大离子在二氧化硅上吸附动力学:聚l-精氨酸的理论与实验互补研究
IF 3.9
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
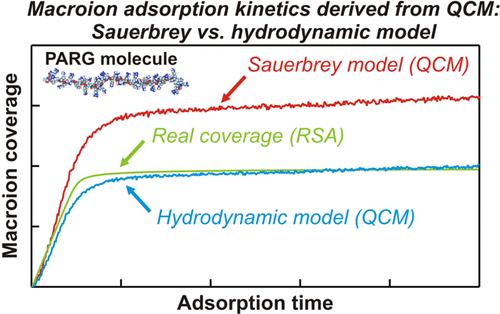
开发了一种全面的方法,可以定量解释聚l-精氨酸(PARG)在固体/电解质界面上的吸附动力学。第一步是对PARG分子构象、轮廓长度和横截面积的物理化学特性进行全原子分子动力学(MD)建模。研究还表明,即使在浓电解质溶液(100 mM NaCl)中,PARG分子也呈现出长径比为36的细长形状。利用MD得到的参数,采用随机顺序吸附法计算了PARG在二氧化硅/电解质界面的吸附动力学。这些预测通过光学反射测量得到了验证。证实了分子在侧面不可逆吸附,其覆盖范围与MD模型预测的PARG分子的细长形状一致。这些理论和实验结果用于解释在不同pH条件下进行的石英晶体微天平测量。综合分析表明,基于流体力学理论的结果表明,高分子分子与传感器之间存在类似润滑的(软)接触,充分反映了吸附动力学。直观使用的Sauerbrey模型的有效性范围也进行了估计。所得结果可用于控制固/液界面上宏离子的吸附。这对于在靶向药物递送中用于生物颗粒固定和纳米胶囊壳形成的支持层的最佳制备至关重要。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Kinetics of Macroion Adsorption on Silica: Complementary Theoretical and Experimental Investigations for Poly-l-arginine
A comprehensive approach enabling a quantitative interpretation of poly-l-arginine (PARG) adsorption kinetics at solid/electrolyte interfaces was developed. The first step involved all-atom molecular dynamics (MD) modeling of physicochemical characteristics yielding PARG molecule conformations, its contour length, and the cross-section area. It was also shown that PARG molecules, even in concentrated electrolyte solutions (100 mM NaCl), assume a largely elongated shape with an aspect ratio of 36. Using the parameters derived from MD, the PARG adsorption kinetics at the silica/electrolyte interface was calculated using the random sequential adsorption approach. These predictions were validated by optical reflectometry measurements. It was confirmed that the molecules irreversibly adsorbed in the side-on orientation and their coverage agreed with the elongated shape of the PARG molecule predicted from the MD modeling. These theoretical and experimental results were used for the interpretation of the quartz crystal microbalance measurements carried out under various pH conditions. A comprehensive analysis unveiled that the results stemming from the hydrodynamic theory postulating a lubrication-like (soft) contact of the macroion molecules with the sensor adequately reflect the adsorption kinetics. The range of validity of the intuitively used Sauerbrey model was also estimated. It was argued that acquired results can be exploited to control macroion adsorption at solid/liquid interfaces. This is essential for the optimum preparation of their supporting layers used for bioparticle immobilization and shell formation at nanocapsules in targeted drug delivery.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


