片段拉帕替尼氨基喹唑啉类似物的设计、合成及抗病毒活性研究
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
COVID-19 对全球健康和经济的严重影响凸显了对创新疗法的迫切需要。最近,一种最初用于治疗乳腺癌的药物拉帕替尼被确定为 SARS-CoV-2 主要蛋白酶(Mpro)的潜在抑制剂,值得进一步研究。在 MD 模拟的指导下,我们利用合理的设计策略,在拉帕替尼结构碎片的基础上开发了新型氨基喹唑啉类似物。初步的计算筛选确定了有希望的候选化合物,并通过 3-4 步的简易工艺合成了这些化合物。体外试验表明,所有类似物对 SARS-CoV-2 感染细胞都有显著的抗病毒效果,其中 Bb1 的 EC50 为 1.10 μM,毒性(50 μM 时为 13.55%)明显低于拉帕替尼。进一步的研究证实,这些类似物能有效抑制 SARS-CoV-2 Mpro,其中 Bb7 的活性最高。MD 模拟显示,Bb7 通过与特定残基的相互作用,在 Mpro 结合袋中实现了稳定性。这些发现表明,氨基喹唑啉类似物有望成为 COVID-19 的候选治疗药物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
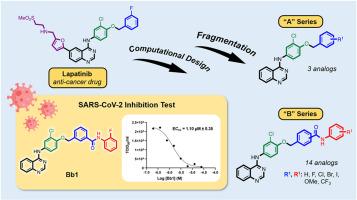
Design, Synthesis, and Antiviral Activity of Fragmented-Lapatinib Aminoquinazoline Analogs towards SARS-CoV-2 Inhibition
The severe impact of COVID-19 on global health and economies highlights the critical need for innovative treatments. Recently, lapatinib, a drug initially used for breast cancer, has been identified as a potential inhibitor of the main protease (Mpro) of SARS-CoV-2, meriting further investigation. Utilizing rational design strategies and guided by MD simulations, we developed novel aminoquinazoline analogs based on fragmented lapatinib's structure. Preliminary computational screenings identified promising candidates, which were synthesized using a concise 3-4 step process. In vitro assays demonstrated notable antiviral efficacy against SARS-CoV-2-infected cells for all analogs, with Bb1 showing an EC50 of 1.10 μM and significantly lower toxicity (13.55% at 50 μM) compared to lapatinib. Further studies confirmed that these analogs effectively inhibit SARS-CoV-2 Mpro, with Bb7 displaying the highest activity. MD simulations revealed that Bb7 achieves stability within the Mpro binding pocket through interactions with specific residues. These findings indicate that aminoquinazoline analogs hold significant promise as therapeutic candidates for COVID-19.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


