新型螺旋环MAT2A抑制剂的发现在mtap无效的异种移植模型中显示出高体内疗效
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
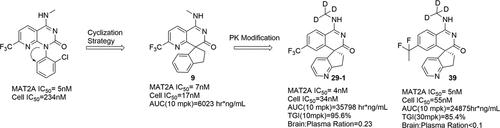
合成致死性为发现针对分子界定的患者群体的下一代精准医学疗法提供了一种强有力的策略。MAT2A抑制剂对S-甲基-5′-硫代腺苷磷酸酶(MTAP)同基因缺失的几种癌症具有合成致死性。在此,我们报告了利用基于结构的药物设计鉴定出的新型 MAT2A 抑制剂,其特点是利用螺旋环来规避 C-N 异构手性。命中化合物 9 在酶活性(IC50 = 7 nM)和 HCT-116 MTAP(-/-)细胞效力(IC50 = 17 nM)方面表现出很高的效力。通过进一步优化,我们发现了两个新的先导化合物:一个是脑穿刺化合物 29-1,另一个是强效但有限的脑穿刺化合物 39。这两种先导化合物都增加了血浆中的药物暴露量,并在去除了 MTAP 的异种移植模型中表现出显著疗效。我们希望找到一种脑渗透剂 MAT2A 抑制剂,为探索 S-腺苷蛋氨酸调节在中枢神经系统中的潜在治疗效果创造新的机会。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Discovery of Novel Spirocyclic MAT2A Inhibitors Demonstrating High In Vivo Efficacy in MTAP-Null Xenograft Models
Synthetic lethality offers a robust strategy for discovering the next generation of precision medicine therapies tailored for molecularly defined patient populations. MAT2A inhibition is synthetically lethal in several cancers that exhibit a homozygous deletion of S-methyl-5′-thioadenosine phosphorylase (MTAP). Herein, we report the identification of novel MAT2A inhibitors featuring a spiral ring to circumvent the C–N atropisomeric chirality utilizing structure-based drug design. The Hit compound 9 exhibited high potency in enzymatic activity (IC50 = 7 nM) and in HCT-116 MTAP(−/−) cell potency (IC50 = 17 nM). Further optimization has led to the identification of two new lead compounds: a brain-penetrant compound, 29–1, and a potent but limited brain-penetrant compound, 39. Both of these lead compounds demonstrate increased plasma drug exposure and exhibit significant efficacy in xenograft models that are depleted of MTAP. We hope that identifying a brain-penetrant MAT2A inhibitor will create new opportunities to explore the potential therapeutic effects of S-adenosylmethionine modulation in the central nervous system.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


