“Cu-Nx”位点驱动选择性开关用于电催化CO2还原
IF 8.3
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
全面了解活性位点周围的化学环境对电化学二氧化碳还原反应(eCO2RR)途径的影响,对于开发大规模应用的先进催化剂至关重要。基于铜离子与邻苯二胺(oPD)、间苯二胺(mPD)和对苯二胺(pPD)等具有邻位、间位和对位两个氨基的苯二胺异构体配位设计的一系列模型催化剂,空间效应可以显著地控制“Cu-N”位点对eCO2RR的选择性。发现Cu-oPD中相邻铜和氮活性位点之间的空间距离增强了*COOH中间体的C-C偶联,从而提高了生成C2H4的选择性。相反,Cu-pPD上*CHO中间体周围的空间静电效应引起的弱范德华相互作用促进了随后的氢化反应,导致CH4的优先合成。然而,Cu-mPD表现出降低的eCO2RR活性,这是由于与速率决定步骤相关的更高的自由能,这主要导致H2的形成。这项研究强调了空间效应驱动的eCO2RR选择性开关的重要作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
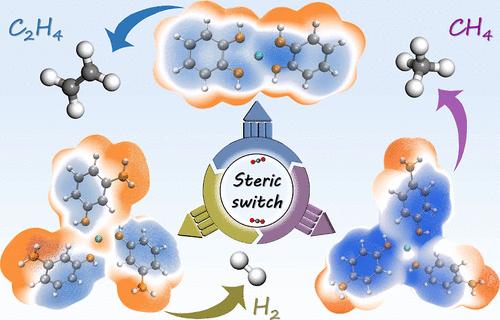
“Cu–Nx” Site-Driven Selectivity Switch for Electrocatalytic CO2 Reduction
The comprehensive understanding of the effect of the chemical environment surrounding active sites on the pathway for the electrochemical carbon dioxide reduction reaction (eCO2RR) is essential for the development of advanced catalysts for large-scale applications. Based on a series of model catalysts engineered by the coordination of copper ions with various isomers of phenylenediamine [i.e., o-phenylenediamine (oPD), m-phenylenediamine (mPD), and p-phenylenediamine (pPD)] featuring two amino groups in ortho-, meta-, and para-positions, the steric effects could significantly govern the selectivity of the “Cu–N” sites for eCO2RR. It was found the steric distance between adjacent copper and nitrogen active sites in Cu-oPD enhanced the C–C coupling of the *COOH intermediate, thereby resulting in increased selectivity for C2H4 production. In contrast, the weak van der Waals interactions arising from steric electrostatic effects surrounding the *CHO intermediate on Cu-pPD facilitated subsequent hydrogenation, leading to the preferential synthesis of CH4. However, Cu-mPD exhibited diminished eCO2RR activity due to a higher free energy associated with the rate-determining step, which primarily led to the formation of H2. This study underscores the significant role of a steric effect-driven selectivity switch for eCO2RR.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


