抗hiv药物Lenacapavir手性三环中间体的非映对和对映选择性化学酶合成
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
尽管其潜力巨大,但在化学酶合成临床重要药物的可扩展的新生物催化转化的开发和实施仍然存在相当大的挑战。我们开发了一种化学酶合成最近开发的抗hiv药物lenacapavir的5/5/3融合三环片段,具有不寻常的手性环丙烷部分。这一发展的关键是一种生物催化剂控制的,高度功能化的乙烯基吡唑底物的完全非对映和对映异构的环丙烷化,可以获得lenacapavir环丙烷的所有四种可能的立体异构体。高通量实验导致血红素依赖性珠蛋白的发现,包括氧化亚氮双加氧酶(NOD)和原珠蛋白(Pgb),作为有前途的环丙烷生物催化剂。定向进化提供了高度的非映对和对映选择性环丙烷化(高达99:1 dr和99:1 e.r)。进一步进行下游化学环化,得到立体化学纯度高的来那卡帕韦5/5/3熔融三环。本文章由计算机程序翻译,如有差异,请以英文原文为准。
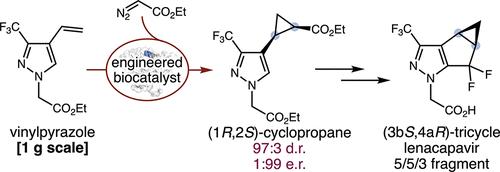
Diastereo- and Enantioselective Chemoenzymatic Synthesis of Chiral Tricyclic Intermediate of Anti-HIV Drug Lenacapavir
Despite its great potential, the development and implementation of scalable new-to-nature biocatalytic transformations in the chemoenzymatic synthesis of clinically significant pharmaceuticals still present a considerable challenge. We developed a chemoenzymatic synthesis of the very recently developed anti-HIV drug lenacapavir’s 5/5/3 fused tricyclic fragment featuring an unusual chiral cyclopropane moiety. Key to this development is a biocatalyst-controlled, fully diastereo- and enantiodivergent cyclopropanation of a highly functionalized vinylpyrazole substrate, granting access to all four possible stereoisomers of lenacapavir cyclopropane. High-throughput experimentation led to the discovery of heme-dependent globins, including nitrous oxide dioxygenase (NOD) and protoglobin (Pgb), as promising cyclopropanation biocatalysts. Directed evolution furnished a highly diastereo- and enantioselective cyclopropanation (up to 99:1 d.r. and 99:1 e.r.). Further developed downstream chemical cyclization afforded the desired lenacapavir 5/5/3 fused tricycle with great stereochemical purity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


