五氯苯胺生物降解P450支架生物催化剂的构建
IF 11.3
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
氯苯胺是一类具有持久性和高毒性的环境污染物,对绿色修复策略提出了重大挑战。虽然P450BM3单加氧酶以其催化惰性C-H键的单氧化能力而闻名,但P450BM3催化所需的昂贵的NAD(P)H和复杂的电子传递系统限制了它们的实际应用。本研究通过战略性地改造P450BM3的活性位点,开创了创新人工生物催化剂的发展。具体来说,用天冬氨酸取代高度保守的苏氨酸268有效地诱导过氧酶活性,从而提高了催化效率。值得注意的是,P450BM3突变体在10-15分钟内对5种氯苯胺(4-氯苯胺、2-氯苯胺、2,4-二氯苯胺、3,4-二氯苯胺和3,5-二氯苯胺)的降解率达到98.38%至99.18%,且不需要NAD(P)H或双功能小分子。UPLC-MS综合降解机理分析证实了这些生物催化剂的显著性能。该研究不仅为P450单加氧酶展示过加氧酶活性提供了一种新的工程方法,而且显著拓宽了其在合成化学和合成生物学中的潜在应用,为更环保、更可持续的修复技术铺平了道路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
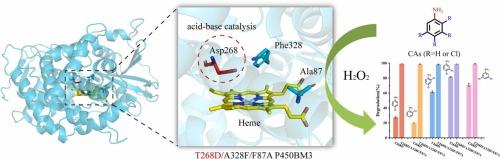
Construction of P450 scaffold biocatalysts for the biodegradation of five chloroanilines
Chloroanilines represent a class of persistent and highly toxic environmental pollutants, posing significant challenges for green remediation strategies. While P450BM3 monooxygenases are renowned for their ability to catalyze the monooxidation of inert C-H bonds, costly NAD(P)H and complex electron transport systems required for P450BM3 catalysis limit their practical applications. This study pioneers the development of innovative artificial biocatalysts by strategically engineering the active site of P450BM3. Specifically, the substitution of the highly conserved threonine 268 with aspartic acid effectively induces peroxygenase activity, allowing for enhanced catalytic efficiency. Remarkably, the engineered P450BM3 mutants achieved degradation rates of 98.38–99.18 % for five chloroanilines (4-chloroaniline, 2-chloroaniline, 2,4-dichloroaniline, 3,4-dichloroaniline, and 3,5-dichloroaniline) in just 10–15 min, all without the need for NAD(P)H or dual-functional small molecules. Comprehensive degradation mechanism analysis via UPLC-MS corroborated the remarkable performance of these biocatalysts. This research not only demonstrates a novel approach for engineering P450 monooxygenases to exhibit peroxygenase activity but also significantly broadens their potential applications in synthetic chemistry and synthetic biology, paving the way for greener and more sustainable remediation technologies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


