高尿酸血症和痛风的肠道微生物组
IF 11.4
1区 医学
Q1 RHEUMATOLOGY
引用次数: 0
摘要
人类通过减少尿酸消除和过量尿酸产生而发展高尿酸血症,从而促进尿酸钠晶体沉积和偶发痛风。正常情况下,大约三分之二的尿酸清除是肾脏的。然而,慢性肾脏疾病(CKD)和其他原因降低肾尿酸消除驱动高尿酸血症在大多数痛风。这对通过肠道消除尿酸盐提出了更高的要求,在肠道中,饮食、嘌呤代谢和微生物群相互作用。尿酸转运到肠道的遗传性损伤是常见的,并促进高尿酸血症,肾尿酸超载,早发和可触及的痛风表型。乳酸菌通过隔离和修饰环境嘌呤,正在研究其抑制饮食诱导的尿酸盐生成和相关痛风发作的潜力。具有里程碑意义的临床前研究最近揭示了多种,专性和兼性厌氧人类和小鼠肠道微生物群(主要是杆状杆菌门)称为嘌呤降解细菌(PDB)的更高容量的尿酸降低作用。PDB中的一个保守基因簇驱动尿酸转化为乳酸或抗炎短链脂肪酸(SCFA)。当小鼠肝脏尿酸酶缺乏以模仿人类尿酸酶缺乏时,微生物群消耗迅速升高盲肠和血清尿酸,通过PDB给药可逆转。在肾功能正常的健康志愿者中,抗生素诱导的肠道微生物群耗损降低了PDB特有的降尿酸基因簇,并提高了粪便尿酸。此外,先前接触无氧覆盖的抗生素与突发痛风风险增加有关。值得注意的是,在痛风队列中观察到包括芽孢杆菌衰竭在内的肠道生态失调。因此,不同肠道细菌菌株的生物化学能力补偿人类在尿酸处置方面的限制,建议采用新的益生菌治疗方法来治疗对耀斑和高尿酸血症都没有充分药理控制的痛风。对于严重CKD来说尤其如此,这限制了常规口服降尿酸药物的选择和最大剂量。本文章由计算机程序翻译,如有差异,请以英文原文为准。
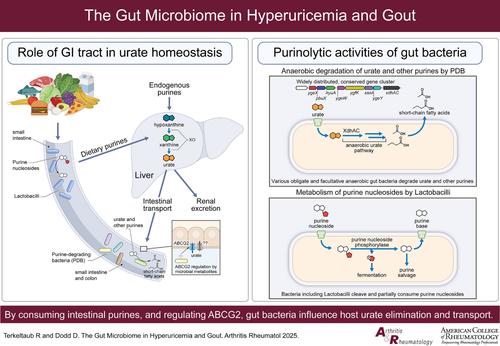
The Gut Microbiome in Hyperuricemia and Gout
Humans develop hyperuricemia via decreased urate elimination and excess urate production, consequently promoting monosodium urate crystal deposition and incident gout. Normally, approximately two thirds of urate elimination is renal. However, chronic kidney disease (CKD) and other causes of decreased renal urate elimination drive hyperuricemia in most with gout. This places more demand on elimination of urate via the gut, where diet, purine metabolism and microbiota intersect. Heritable impairment of urate transport into the gut is common, and promotes hyperuricemia, renal urate overload, and early onset and palpable tophaceous gout phenotypes. Lactobacilli, by sequestering and modifying ambient purines, are being studied for the potential to suppress diet-induced urate generation and associated gout flares. Landmark preclinical studies recently revealed much higher-capacity urate-lowering effects of diverse, obligate and facultative anaerobic human and murine gut microbiota (predominantly of Bacillota phylum) termed purine-degrading bacteria (PDB). A conserved gene cluster in PDB drives urate conversion to lactate or anti-inflammatory short chain fatty acids (SCFA). When mice are rendered deficient in hepatic uricase to mimic human uricase absence, microbiota depletion rapidly elevates both cecal and serum urate, reversible by PDB administration. In healthy human volunteers with normal renal function, antibiotic-induced gut microbiota depletion, decreases the urate-lowering gene cluster unique to PDB and elevates fecal urate. Also, prior exposure to antibiotics with anaerobic coverage has been linked to heightened incident gout risk. Notably, intestinal dysbiosis that includes Bacillota depletion has been observed in gout cohorts. Therefore, the capacity of diverse gut bacterial strains to biochemically compensate for human limits in urate disposition suggests novel probiotic treatment approaches for gout with inadequate pharmacologic control of both flares and hyperuricemia. This is particularly so for severe CKD, which limits the options and maximal doses for use of conventional oral urate-lowering drugs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Arthritis & Rheumatology
RHEUMATOLOGY-
CiteScore
20.90
自引率
3.00%
发文量
371
期刊介绍:
Arthritis & Rheumatology is the official journal of the American College of Rheumatology and focuses on the natural history, pathophysiology, treatment, and outcome of rheumatic diseases. It is a peer-reviewed publication that aims to provide the highest quality basic and clinical research in this field. The journal covers a wide range of investigative areas and also includes review articles, editorials, and educational material for researchers and clinicians. Being recognized as a leading research journal in rheumatology, Arthritis & Rheumatology serves the global community of rheumatology investigators and clinicians.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


