钴基反三明治型配合物中平面π-芳香Bi5−环的分离
IF 19.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
单环π芳香族化合物在自然科学的几乎所有领域都无处不在,它们是工业生产过程中的合成物,是催化或传感金属复合物的配体,也是具有生物活性的分子。平面有机环通过一组不可定位的 (4n + 2)π 电子克服电子缺乏的特殊方式脱颖而出。相比之下,全金属芳香族单环仍然很少见,因为金属原子更愿意与多键原子形成簇。这限制了相应化合物在化学合成或创新材料方面的知识和潜力。在此,我们报告了 (C5H5)- 的最重类似物 Bi5- 的成功生成。它作为[{IMesCo}2(µ,η5:η5-Bi5)](1)的配体,是通过 (TlBi3)2- 与[(IMes)2CoCl](其中 IMes 是双(1,3-(2,4,6-三甲基苯基))咪唑-2-亚基)在正二氟苯中反应而实现的。经 µ-SQUID 测量和密度泛函理论验证,化合物 1 是混价 Co0/CoI,并以反桑威奇型方式嵌入了平面 Bi5- 循环。捕获 Bi5- 标志着全金属芳香分子化学的一个里程碑,并定义了芳香化合物的一个新时代。本文章由计算机程序翻译,如有差异,请以英文原文为准。

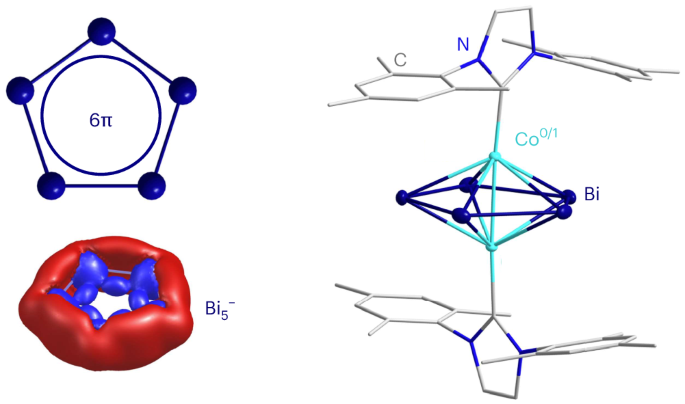
Isolation of a planar π-aromatic Bi5− ring in a cobalt-based inverse-sandwich-type complex
Monocyclic π-aromatic compounds are ubiquitous throughout almost all fields of natural sciences—as synthons in industrial processes, as ligands of metal complexes for catalysis or sensing and as bioactive molecules. Planar organocycles stand out through their specific way of overcoming electron deficiency by a non-localizable set of (4n + 2)π electrons. By contrast, all-metal aromatic monocycles are still rare, as metal atoms prefer to form clusters with multiply bonded atoms instead. This limits the knowledge and potential of corresponding compounds in chemical syntheses or for innovative materials. Here we report the successful generation of Bi5−, the heaviest analogue of (C5H5)−. Its use as a ligand in [{IMesCo}2(µ,η5:η5-Bi5)] (1) was realized by reacting (TlBi3)2− with [(IMes)2CoCl] (where IMes is bis(1,3-(2,4,6-trimethylphenyl))imidazol-2-ylidene) in ortho-difluorobenzene. Compound 1 is mixed-valence Co0/CoI as verified by µ-SQUID measurements and density functional theory, and embeds the planar Bi5− cycle in an inverse-sandwich-type manner. Capturing Bi5− represents a landmark in the chemistry of all-metal aromatic molecules and defines a new era for aromatic compounds. All-metal aromatic monocycles are still rare, in contrast to their ubiquitous organic counterparts, because metal atoms tend to form clusters with multiply bonded atoms instead. Now a planar aromatic Bi5− ring has been synthesized as part of a mixed-valence Co0/CoI inverse-sandwich-type complex.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature chemistry
化学-化学综合
CiteScore
29.60
自引率
1.40%
发文量
226
审稿时长
1.7 months
期刊介绍:
Nature Chemistry is a monthly journal that publishes groundbreaking and significant research in all areas of chemistry. It covers traditional subjects such as analytical, inorganic, organic, and physical chemistry, as well as a wide range of other topics including catalysis, computational and theoretical chemistry, and environmental chemistry.
The journal also features interdisciplinary research at the interface of chemistry with biology, materials science, nanotechnology, and physics. Manuscripts detailing such multidisciplinary work are encouraged, as long as the central theme pertains to chemistry.
Aside from primary research, Nature Chemistry publishes review articles, news and views, research highlights from other journals, commentaries, book reviews, correspondence, and analysis of the broader chemical landscape. It also addresses crucial issues related to education, funding, policy, intellectual property, and the societal impact of chemistry.
Nature Chemistry is dedicated to ensuring the highest standards of original research through a fair and rigorous review process. It offers authors maximum visibility for their papers, access to a broad readership, exceptional copy editing and production standards, rapid publication, and independence from academic societies and other vested interests.
Overall, Nature Chemistry aims to be the authoritative voice of the global chemical community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


