磁共振成像后患者尿液中钆的电化学过滤
IF 8.2
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
近几十年来,钆基造影剂在磁共振成像(MRI)中的广泛使用导致了对钆的需求不断增长,同时也因其直接排入废水系统而引发了环境问题。为此,我们开发了一种电化学过滤方法,用于在造影剂增强核磁共振成像后从患者尿液中回收钆。这种方法是对传统的真空过滤设备进行改装,在滤膜中引入电极,在滤膜中形成 5 kV/m 的强电场和陡峭的三区 pH 梯度。这些电场和 pH 值梯度促进了钆基造影剂的解离,释放出 GdIII 离子,GdIII 及其配体发生电泳分离,最终 GdIII 以 GdPO4 和 Gd(OH)3 的形式沉淀并截留在滤膜上。我们使用钆喷酸二葡胺(GdDTPA)作为钆基造影剂模型,对人工尿液和真实尿液样本的钆捕获效率达到了 70%。对于大环钆基造影剂,例如钆喷酸葡胺(GdDOTA),由于大环造影剂的解离动力学较慢,钆的捕获效率降至 25.4%。不过,在电化学过滤之前,让大环造影剂在酸性环境中预解离,可以将捕获效率提高到 40%。滤膜上截留的钆可以通过马弗炉中的热处理回收。经过热处理后,从真实尿液样本中回收的钆主要被鉴定为 GdPO4。这种电化学过滤设计为从对比增强核磁共振成像扫描中回收钆提供了一种直接而实用的方法,既满足了对钆日益增长的需求,又有助于减轻人们对地表水中钆污染的担忧。本文章由计算机程序翻译,如有差异,请以英文原文为准。
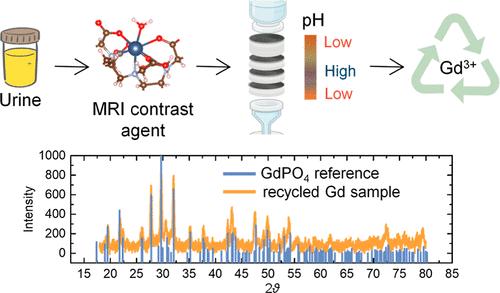
Electrochemical Filtration of Gadolinium from Patient Urine after Magnetic Resonance Imaging
The widespread use of gadolinium-based contrast agents for magnetic resonance imaging (MRI) in recent decades has led to a growing demand for Gd and raised environmental concerns due to their direct discharge into wastewater systems. In response, we developed an electrochemical filtration method to recover Gd from patient urine following contrast-enhanced MRI. This method involves modifying a conventional vacuum filtration apparatus by introducing electrodes into the filter membrane, creating a strong electric field of ∼5 kV/m and a steep three-zone pH gradient within the filter membrane. These electric and pH fields facilitate the dissociation of Gd-based contrast agents, releasing GdIII ions, electrophoretic separation of GdIII and its ligand, and eventually precipitation and trapping of GdIII as GdPO4 and Gd(OH)3 on the filter membrane. Using gadopentetate dimeglumine (GdDTPA) as a model Gd-based contrast agent, we achieved a Gd trapping efficiency of ∼70% for artificial and real urine samples. For macrocyclic Gd-based contrast agents such as gadoterate meglumine (GdDOTA), the Gd trapping efficiency decreased to 25.4% due to the slow dissociation kinetics of macrocyclic contrast agents. However, the trapping efficiency can be improved to ∼40% by allowing the macrocyclic contrast agent to predissociate in an acidic environment before electrochemical filtration. The Gd trapped on the filter membrane can be recovered by thermal treatment in a muffle furnace. After thermal treatment, the reclaimed Gd from the real urine sample was primarily identified as GdPO4. This electrochemical filtration design offers a straightforward and practical approach to recovering Gd from contrast-enhanced MRI scans, addressing the increasing demand for Gd and helping alleviate concerns about Gd contamination in surface water.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


