通过单分子传导从双键中识别碳碳三键
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
分子尺度电子学侧重于理解和利用单个分子的电荷传输。一个关键问题是单分子的电荷输运能力,其特征是电流衰减。我们利用扫描隧道显微镜和非接触原子力显微镜观察了不同碳碳键序共轭聚合物的现场形成过程。虽然碳碳双键和三键表现出相似的电子特征,但单分子电导测量显示出基于不同共轭水平的不同特征。这些发现得到了密度泛函理论计算的支持,表明更高的键序导致更大的电子密度和更对称的分子轨道,从而导致更大的传输速率和更刚性的前沿轨道。因此,这有助于更高的电导和更低的衰减常数。这些发现增强了对分子电子学中键序的理解,并将促进单分子器件的发展和纳米级电路的应用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
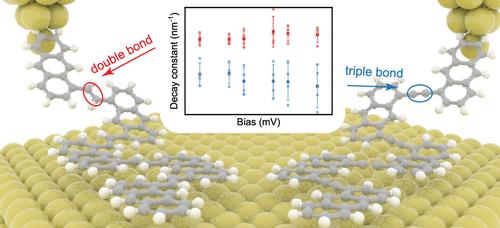
Identifying Carbon–Carbon Triple Bonds from Double Bonds via Single-Molecule Conductance
Molecular-scale electronics focuses on understanding and utilizing charge transport through individual molecules. A key issue is the charge transport capability of a single molecule characterized by current decay. We visualize the on-site formation of conjugated polymers with varying carbon–carbon bond orders by using scanning tunneling microscopy and noncontact atomic force microscopy. Although carbon–carbon double bonds and triple bonds exhibit similar electronic characteristics, single-molecule conductance measurements reveal distinct features based on different levels of conjugation. These findings, supported by density functional theory calculations, indicate that a higher bond order results in greater electron density and more symmetric molecular orbitals, leading to larger transmission rates and more rigid frontier orbitals. Consequently, this contributes to a higher conductance and a lower decay constant. These findings enhance the understanding of bond orders in molecular electronics and should facilitate the development of single-molecule devices and the applications of nanoscale circuitry.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


