重塑烯还原酶的底物结合袋以增强和倒置立体选择性:立体互补手性GABA衍生物的简明途径
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
尽管有许多天然的和工程的烯还原酶(er),但由于底物接受性差,特别是互补立体选择性不足,er对映互补合成的立体受阻分子仍然受到限制。在此,我们通过半设计重塑了真贝酵母SeER的底物结合袋,使其能够立体互补氢化取代的β-氰基肉桂酸酯。与野生型相比,该突变体具有更高的活性(高达161倍)和催化效率(高达358倍),显示出合成各种具有高立体选择性(高达99% ee)的手性β-氰基酯的潜力。分子动力学模拟表明,变体具有优异的催化性能的关键是调节良好的底物结合口袋,它加强和稳定了底物识别。此外,我们阐明了SeER变体在通过化学酶级联反应不对称合成手性GABA衍生物(如Phenibut, Baclofen和Tolibut)中的实用性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
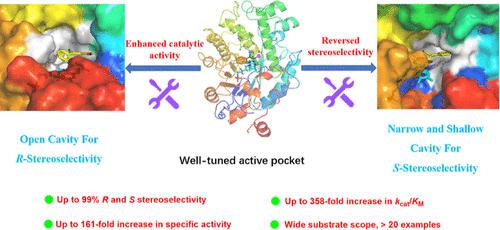
Reshaping the Substrate-Binding Pocket of Ene-Reductase for Enhanced and Inverted Stereoselectivity: A Concise Access to the Stereocomplementary Chiral GABA Derivatives
Despite the availability of numerous natural and engineered ene-reductases (ERs), enantiocomplementary synthesis of the sterically hindered molecules by ERs is still limited by poor substrate acceptance, particularly due to the insufficient complementary stereoselectivity. Herein, we reshaped the substrate-binding pocket of SeER from Saccharomyces eubayanus through semirational design, enabling ERs capable of stereocomplementary hydrogenating of the challenging substituted β-cyano cinnamic esters. Compared to the wild type, the variants exhibited enhanced activity (up to 161-fold) and catalytic efficiency kcat/KM (up to 358-fold), displaying potential in synthesizing various chiral β-cyano esters with high stereoselectivity (up to 99% ee). Molecular dynamics simulations demonstrated that the key for the superior catalytic performance of variants is the well-tuned substrate-binding pocket, which strengthens and stabilizes substrate recognition. Furthermore, we elucidated the practicality of the SeER variants in asymmetric synthesis of the chiral GABA derivatives (e.g., Phenibut, Baclofen, and Tolibut) via chemo-enzymatic cascade reactions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


