含硫锑酸盐的水用纳米晶碘化钾(铁基层状双氢氧化物)有效处理
IF 7.3
2区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
摘要
由于锑(Sb)的高毒性和高流动性,环境中来自自然和人为来源的锑浓度升高引起了全球关注。值得注意的是,硫代锑物种(如三硫代锑酸盐和四硫代锑酸盐)的形成会增加锑在水生系统中的释放量,在硫化地热水等还原性环境中,硫代锑物种可能占主导地位。纳米晶 iowaite 是一种适合吸附有害阴离子的吸附剂,但迄今为止尚未用于处理含硫锑酸盐的水体,本研究通过批量吸附实验研究了纳米晶 iowaite 从水溶液中去除硫代锑酸盐的情况。根据动力学和等温线吸附模型的拟合,以及利用 XRD 和 XPS 分析对 iowaite 与含硫代锑酸盐溶液反应前后的结构进行比较,发现溶液中的硫代锑酸盐与 iowaite 层间区域的氯化物之间的阴离子交换,以及吸附的硫代锑酸盐与 iowaite 层中的铁原子之间的内球络合,是 iowaite 高效去除水体中的硫代锑酸盐的原因。此外,普通阴离子(SO42-、Cl- 和 NO3-)或锑氧阴离子(锑酸盐和锑酸盐)在较大浓度范围内的存在对硫代锑酸酯的吸附影响甚微,而高浓度砷氧阴离子(亚砷酸盐和砷酸盐)的加入则明显抑制了硫代锑酸酯的去除。尽管如此,在环境相关条件下,iowaite 仍然是硫代锑酸酯的强力清除剂,因此有望在未来大规模处理可能富含硫代锑酸酯的地热水、缺氧地下水和采矿废水。本文章由计算机程序翻译,如有差异,请以英文原文为准。
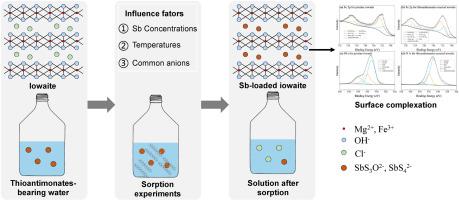
Effective treatment of thioantimonates-bearing waters by nanocrystalline iowaite, an iron-based layered double hydroxide
Elevated concentrations of antimony (Sb) in the environment originating from natural and anthropogenic sources are of global concern due to their high toxicity and mobility. Notably, the formation of thioantimony species (e.g. tri- and tetra-thioantimonates) enhances Sb release into aquatic systems, and they may dominate Sb species in reducing environments, such as sulfidic geothermal waters. In this study, batch sorption experiments were conducted to investigate thioantimonates removal from aqueous solution by nanocrystalline iowaite, a sorbent suitable for incorporating hazardous anions but not used for treating thioantimonates-bearing waters up to now. Based on the fits of kinetics and isotherm sorption models as well as the structural comparison of iowaite before and after its reaction with thioantimonates-bearing solutions by use of XRD and XPS analyses, both the anion exchange between thioantimonates in solution and chloride in the interlayer regions of iowaite and the inner-sphere complexation of sorbed thioantimonates with iron atoms located in iowaite layers were found to be responsible for the efficient removal of aqueous thioantimonates by iowaite. Moreover, the presence of common anions (SO42−, Cl−, and NO3−) or Sb oxyanions (antimonite and antimonate) over a wide range of concentrations had little effect on thioantimonates sorption, whereas the addition of arsenic oxyanions (arsenite and arsenate) with high concentrations exhibited a clear inhibition of thioantimonates removal. Nevertheless, iowaite is still a strong scavenger of thioantimonates under environmentally relevant conditions and therefore holds promise for future large-scale treatment of geothermal waters, anoxic groundwaters, and mining wastewaters that are potentially rich in thioantimonates.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Environmental Pollution
环境科学-环境科学
CiteScore
16.00
自引率
6.70%
发文量
2082
审稿时长
2.9 months
期刊介绍:
Environmental Pollution is an international peer-reviewed journal that publishes high-quality research papers and review articles covering all aspects of environmental pollution and its impacts on ecosystems and human health.
Subject areas include, but are not limited to:
• Sources and occurrences of pollutants that are clearly defined and measured in environmental compartments, food and food-related items, and human bodies;
• Interlinks between contaminant exposure and biological, ecological, and human health effects, including those of climate change;
• Contaminants of emerging concerns (including but not limited to antibiotic resistant microorganisms or genes, microplastics/nanoplastics, electronic wastes, light, and noise) and/or their biological, ecological, or human health effects;
• Laboratory and field studies on the remediation/mitigation of environmental pollution via new techniques and with clear links to biological, ecological, or human health effects;
• Modeling of pollution processes, patterns, or trends that is of clear environmental and/or human health interest;
• New techniques that measure and examine environmental occurrences, transport, behavior, and effects of pollutants within the environment or the laboratory, provided that they can be clearly used to address problems within regional or global environmental compartments.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


