肠道菌群失调通过炎症参与十溴联苯醚诱导的骨稳态紊乱
IF 7.3
2区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
摘要
BDE-209 与不良健康后果存在因果关系。然而,有关其对骨骼稳态影响的研究却相对缺乏。本研究采用体外和体内模型,研究了 BDE-209 暴露与骨骼健康之间的关系及其内在机制。在动物研究中,雌性 SD 大鼠连续 60 天摄入 BDE-209。对骨矿密度、骨微结构、肠道微生物群和炎症标志物进行了测量。此外,用含有相关剂量的 BDE-209 或丁酸钠的培养基处理 THP-1 细胞衍生的巨噬细胞。测量了 M1 巨噬细胞标志物、破骨细胞标志物和炎症细胞因子的表达。然后用破骨细胞条件培养基诱导巨噬细胞,以评估 BDE-209 对其分化为破骨细胞的影响。结果显示,暴露组的肱骨骨密度降低,破骨细胞活性增强,IL-1β、TNF-α、IL-6 上调,PGC-1α/NAD+/cGAS-STING 被激活。16s 测序显示,BDE-209 破坏了肠道微生物群的丰度,特别是减少了拉赫诺斯皮拉菌科(Lachnospiraceae)的数量。在体外,BDE-209 可刺激巨噬细胞分化出更多破骨细胞并激活 cGAS-STING 通路,而丁酸钠可抑制这些效应。本研究揭示了肠道微生物群失调通过炎症老化参与了 BDE-209 诱导的骨稳态紊乱,而丁酸钠可减轻这种影响。总之,这项研究为预防和治疗与 BDE-209 暴露相关的骨质疏松症提供了研究数据。本文章由计算机程序翻译,如有差异,请以英文原文为准。

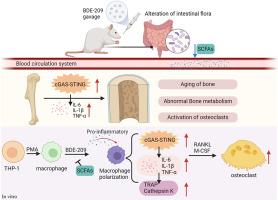
Gut microbiota dysbiosis involved in decabromodiphenyl ether-induced bone homeostasis disorder through inflammaging
BDE-209 has a causal relationship with adverse health outcomes. However, research on its effect on bone homeostasis is relatively lacking. This study examined the relationship between BDE-209 exposure and bone health, as well as the underlying mechanisms, using both in vitro and in vivo models. In animal studies, female SD rats were administered BDE-209 for 60 days. Bone mineral density, bone microstructure, gut microbiota, and inflammaging markers were measured. Furtherly, THP-1 cell-derived macrophages were treated with a culture medium containing population-relevant dose of BDE-209 or sodium butyrate. The expression of M1 macrophage markers, osteoclast markers, and inflammatory cytokines was measured. Then macrophages were induced by osteoclast conditioned medium to evaluate the effect of BDE-209 on their differentiation into osteoclasts. Results showed reduced humeral bone density, enhanced osteoclast activity, upregulation of IL-1β, TNF-α, IL-6, and activation of PGC-1α/NAD+/cGAS-STING in the exposed group. 16s sequencing revealed that BDE-209 disrupts the abundance of the gut microbiota, notably a reduction in Lachnospiraceae. In vitro, BDE-209 can stimulate macrophages to differentiate more osteoclasts and activate the cGAS-STING pathway, while sodium butyrate can inhibit these effects. This study reveals that gut microbiota dysbiosis is involved in BDE-209-induced bone homeostasis disorder through inflammatory aging and sodium butyrate can mitigate this effect. Overall, this study provides research data for the precaution and treatment of osteoporosis associated with BDE-209 exposure.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Environmental Pollution
环境科学-环境科学
CiteScore
16.00
自引率
6.70%
发文量
2082
审稿时长
2.9 months
期刊介绍:
Environmental Pollution is an international peer-reviewed journal that publishes high-quality research papers and review articles covering all aspects of environmental pollution and its impacts on ecosystems and human health.
Subject areas include, but are not limited to:
• Sources and occurrences of pollutants that are clearly defined and measured in environmental compartments, food and food-related items, and human bodies;
• Interlinks between contaminant exposure and biological, ecological, and human health effects, including those of climate change;
• Contaminants of emerging concerns (including but not limited to antibiotic resistant microorganisms or genes, microplastics/nanoplastics, electronic wastes, light, and noise) and/or their biological, ecological, or human health effects;
• Laboratory and field studies on the remediation/mitigation of environmental pollution via new techniques and with clear links to biological, ecological, or human health effects;
• Modeling of pollution processes, patterns, or trends that is of clear environmental and/or human health interest;
• New techniques that measure and examine environmental occurrences, transport, behavior, and effects of pollutants within the environment or the laboratory, provided that they can be clearly used to address problems within regional or global environmental compartments.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


