醋酸钾水合物的组成、结构和热性能
IF 4.7
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
晶体结构决定了化合物和材料的性质,尽管人们可以找到简单但与工业相关的化合物,如醋酸钾(KOAc)及其水合物,由于缺乏结构数据,其性质甚至组成仍然被误解。本研究首次确定了KOAc多晶和水合物的晶体结构。晶体水合物的含水量得到了可靠的测定,揭示了两种新的相3KOAc·2H2O和KOAc·xH2O (x = 0.38-0.44),而不是一个世纪以来被认为是KOAc在室温下主要水合形式的“半水合物”和“半水合物”。明确了相变的数量和性质,并通过变温x射线衍射和热分析对水合-脱水过程进行了详细研究。本文章由计算机程序翻译,如有差异,请以英文原文为准。
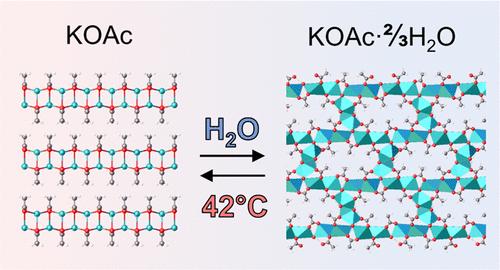
Composition, Structure, and Thermal Properties of Potassium Acetate Hydrates
The crystal structure determines the properties of compounds and materials, although one can find simple yet industrially relevant compounds such as potassium acetate (KOAc) and its hydrates for which the properties and even the composition still remain misunderstood, owing to the lack of structural data. In this study, the crystal structures of KOAc polymorphs and hydrates were determined for the first time. The water content in the crystal hydrates was reliably determined revealing two new phases 3KOAc·2H2O and KOAc·xH2O (x = 0.38–0.44), instead of the “sesquihydrate” and “hemihydrate” that for a century were believed to be the main hydrated forms of KOAc at ambient temperature. The number and nature of phase transitions were clearly established, and the hydration–dehydration processes were studied in detail by variable-temperature X-ray diffraction and thermal analyses.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


