钒化合物的水解、配体交换和氧化还原性质:溶液转化在生物、治疗和环境应用中的意义
IF 55.8
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
钒是一种具有重要工业、技术、生物和生物医学应用的过渡金属,广泛存在于环境和生物体内。综述了钒化合物在蛋白质、核酸、脂质和代谢物存在下在温和生理条件下发生的不同反应。在环境中,钒是自然存在的或通过人为来源存在的,后者由于vc在大气和含水层中的分散而对环境产生影响。钒具有多种化学性质,氧化态可以相互转换,配位数和几何形状可以改变,并且能够形成具有不同核和结构的多氧化钒酸盐。如果VC被添加到含水的环境中,它可以发生水解、配体交换、氧化还原和其他类型的变化,这取决于钒的条件和形态化学。重要的是,溶液可能与引入系统的VC不同,并随浓度而变化。本文描述了钒的氧化还原、水解和配体交换化学反应,以及pH、浓度、盐、特定溶质、生物分子和vc对物种形成的影响。我们这项工作的目标之一是强调评估VC物种形成的必要性,以便确定有益或有毒的物种,并阐明其作用机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
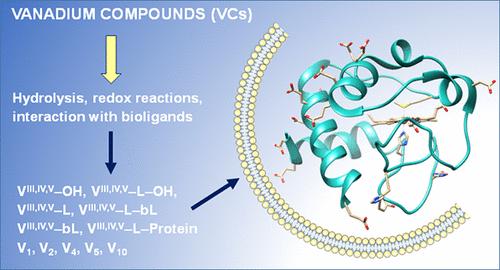
Hydrolysis, Ligand Exchange, and Redox Properties of Vanadium Compounds: Implications of Solution Transformation on Biological, Therapeutic, and Environmental Applications
Vanadium is a transition metal with important industrial, technological, biological, and biomedical applications widespread in the environment and in living beings. The different reactions that vanadium compounds (VCs) undergo in the presence of proteins, nucleic acids, lipids and metabolites under mild physiological conditions are reviewed. In the environment vanadium is present naturally or through anthropogenic sources, the latter having an environmental impact caused by the dispersion of VCs in the atmosphere and aquifers. Vanadium has a versatile chemistry with interconvertible oxidation states, variable coordination number and geometry, and ability to form polyoxidovanadates with various nuclearity and structures. If a VC is added to a water-containing environment it can undergo hydrolysis, ligand-exchange, redox, and other types of changes, determined by the conditions and speciation chemistry of vanadium. Importantly, the solution is likely to differ from the VC introduced into the system and varies with concentration. Here, vanadium redox, hydrolytic and ligand-exchange chemical reactions, the influence of pH, concentration, salt, specific solutes, biomolecules, and VCs on the speciation are described. One of our goals with this work is highlight the need for assessment of the VC speciation, so that beneficial or toxic species might be identified and mechanisms of action be elucidated.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Reviews
化学-化学综合
CiteScore
106.00
自引率
1.10%
发文量
278
审稿时长
4.3 months
期刊介绍:
Chemical Reviews is a highly regarded and highest-ranked journal covering the general topic of chemistry. Its mission is to provide comprehensive, authoritative, critical, and readable reviews of important recent research in organic, inorganic, physical, analytical, theoretical, and biological chemistry.
Since 1985, Chemical Reviews has also published periodic thematic issues that focus on a single theme or direction of emerging research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


