氢氧化钠焙烧-水浸直接从α-锂辉石中提取锂
IF 13.2
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
从锂的主要矿物来源(即锂辉石)中提取锂的传统方法涉及复杂的高耗能过程,包括通过高温煅烧将天然存在的α-锂辉石转化为可浸出的β-锂辉石,然后进行硫酸焙烧,最后进行水浸。为了解决与传统方法相关的经济和环境挑战,本文提出了一种正在申请专利的从α-锂辉石中直接提取锂的工艺。该方法采用氢氧化钠低温焙烧将α-锂辉石转化为水溶性含锂相,并通过水浸回收锂。通过对工艺化学、热力学、动力学等方面的研究,进一步优化工艺参数,最大限度地提高锂的回收率。焙烧过程在NaOH熔点(318 °C)左右促进了碱-硅反应,生成了水溶性LiNaSiO4相,省去了高温煅烧和酸焙烧过程。焙烧反应遵循缩心模型。放热水浸反应表现出快速的动力学,在室温下1分钟内实现最大的锂回收。在水浸过程中,NaOH自由基的再生产生了pH值约为13的产物溶液,减少了下游净化过程中的化学消耗。在此基础上,建立了逆流浸出工艺流程并进行了验证。优化后的两段氢氧化钠焙烧水浸工艺可使锂回收率达到99% %以上。这种从α-锂辉石中直接提取锂的方法提供了一种可持续的解决方案,在满足日益增长的锂需求的同时,最大限度地减少了传统提取过程的环境足迹。本文章由计算机程序翻译,如有差异,请以英文原文为准。
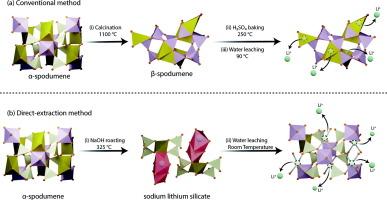
Direct lithium extraction from α-Spodumene using NaOH roasting and water leaching
The conventional method for extracting Li from the primary mineral source of Li (i.e., spodumene) involves complex and energy-intensive processes, including the conversion of naturally occurring α-spodumene to leachable β-spodumene through high-temperature calcination, followed by sulfuric acid baking, and water leaching. To address the economic and environmental challenges associated with the conventional method, this paper presents a patent-pending process for the direct extraction of Li from α-spodumene. This innovative method entails low-temperature roasting with NaOH to convert α-spodumene to water-soluble Li-bearing phases, from which Li is recovered through water leaching. This paper studies the process chemistry, thermodynamics, and kinetics, and further optimizes its parameters to maximize Li recovery. The roasting process promoted the alkali-silica reaction at about the melting temperature of NaOH (318 °C), producing water-soluble LiNaSiO4 phase, eliminating the need for high-temperature calcination and acid-baking processes. The roasting reaction followed shrinkage core model. The exothermic water leaching reaction exhibited fast kinetics, achieving maximum Li recovery within one minute at room temperature. The regeneration of NaOH radicals during the water leaching yielded product solution with a pH of approximately 13, reducing chemical consumption in downstream purification. Based on the obtained results, a process flowsheet incorporating countercurrent water leaching was developed and validated. The optimized proposed two-stage NaOH roasting and water leaching process resulted in over 99 % Li recovery. This direct Li extraction method from α-spodumene offers a sustainable solution, with significant potential to meet the growing demand for lithium while minimizing the environmental footprint of the conventional extraction process.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


