从污泥衍生的煤焦中回收磷:平衡磷回收、重金属伴随浸出和残余煤焦利用
IF 10
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
水热碳化结合湿法化学法处理污泥可产生有价值的烃类和磷。但需要进一步优化磷回收、重金属浸出和残焦利用之间的平衡,以提高整体工艺效益。在本研究中,采用HCl、H2SO4、柠檬酸,在0.1 ~ 1.0 M酸浓度下,液固比在50 ~ 500 mL/g之间,进行了10 ~ 1440 min的氢炭酸浸。采用单因素实验、响应面法和各种表征技术,定量评估了酸浸对磷和重金属浸出率的影响,以及剩余烃类的残留率和性质。结果表明,浸出参数直接影响磷的浸出速率。在酸浓度0.5 M、液固比500 mL/g条件下,HCl和H2SO4在60 min内达到相应的最大磷浸出速率,而使用柠檬酸则需要360 min。以H2SO4作为萃取剂时,在较宽的浸出时间和酸浓度范围内均可保持相应的最大磷浸出速率。适度的酸浓度、较长的浸出时间、较高的液固比导致重金属伴随浸出水平升高。与HCl和柠檬酸相比,H2SO4作为萃取剂对重金属的浸出率较低,对浸出参数的敏感性较低。氢炭的残留率为59.3% ~ 77.3%,以H2SO4和HCl作为萃取剂的残留率高于柠檬酸。酸浸会改变剩余烃类的性质,使其比表面积和孔数减少,但含氧官能团和热值增加,影响其后续利用。最后,根据上述结果和可能的工艺方案,讨论了最佳酸浸工艺参数的选择。研究结果可为污泥源烃类磷回收和资源化利用途径的选择提供参考。本文章由计算机程序翻译,如有差异,请以英文原文为准。
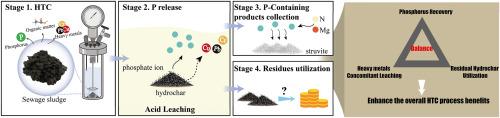
Phosphorus recovery from sewage sludge-derived hydrochar: Balancing phosphorus recovery, heavy metal concomitant leaching and residual hydrochar utilization
Hydrothermal carbonization combined with wet chemical methods for treating sewage sludge produce valuable hydrochar and phosphorus. However, further investigation is needed to optimize the balance among phosphorus recovery, heavy metal leaching, residual hydrochar utilization to enhance overall process benefits. In this study, acid leaching on hydrochar was conducted using HCl, H2SO4, citric acid at 0.1–1.0 M acid concentration for 10–1440 min, the liquid-to-solid ratio between 50 and 500 mL/g. Employing single-factor experiments, response surface methodology, various characterization techniques, the impacts of acid leaching on the leaching rates of phosphorus and heavy metals, along with residual rate and properties of the remaining hydrochar, were quantitatively assessed. The results indicate leaching parameters directly impact the phosphorus leaching rate. HCl and H2SO4 achieve their corresponding maximum phosphorus leaching rate within 60 min at acid concentration 0.5 M, liquid-to-solid ratio 500 mL/g, while using citric acid requires 360 min. When H2SO4 is used as the extractant, the corresponding maximum phosphorus leaching rate can be maintained within a wider leaching time and acid concentration range. A moderate acid concentration, prolonged leaching time, higher liquid-to-solid ratio lead to increased levels of heavy metal concomitant leaching. Compared to HCl and citric acid, using H2SO4 as extractant results in lower heavy metal leaching and lower sensitivity to leaching parameters. The residual rates of hydrochar range from 59.3% to 77.3%, with H2SO4 and HCl as extractant yielding higher residual rates than citric acid. The properties of the remaining hydrochar were altered by acid leaching, resulting in a decrease in specific surface area and pore number, but an increase in oxygen-containing functional groups and calorific value, which affects its subsequent utilization. Finally, based on the aforementioned results and potential process scenarios, the selection of optimal acid leaching parameters was discussed. The results provide reference for the selection of phosphorus recovery and resource utilization pathway based on sludge-derived hydrochar.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Cleaner Production
环境科学-工程:环境
CiteScore
20.40
自引率
9.00%
发文量
4720
审稿时长
111 days
期刊介绍:
The Journal of Cleaner Production is an international, transdisciplinary journal that addresses and discusses theoretical and practical Cleaner Production, Environmental, and Sustainability issues. It aims to help societies become more sustainable by focusing on the concept of 'Cleaner Production', which aims at preventing waste production and increasing efficiencies in energy, water, resources, and human capital use. The journal serves as a platform for corporations, governments, education institutions, regions, and societies to engage in discussions and research related to Cleaner Production, environmental, and sustainability practices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


