用不混相钽合金化提高铜的CO氧化性能
IF 8.3
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
铜钽(Cu-Ta)非混相合金纳米颗粒(NPs)由于其优异的纳米结构稳定性和高温蠕变性能,一直是结构材料领域广泛研究的课题。然而,Cu由于其丰富的价态变化,也是一种高活性的氧化催化剂。在这项研究中,我们首次通过湿法共还原获得了均匀的CuxTa1-x (x = 0.5, 0.7, 0.9, 1)纳米颗粒,平均粒径约为30 nm。CuxTa1-x /TiO2 (x = 0.5, 0.7, 0.9)的CO氧化活性均高于Cu/TiO2,其中Cu0.7Ta0.3/TiO2表现出较好的性能。氢程序升温还原实验表明,Cu0.7Ta0.3/TiO2具有增强的氧化还原性能。动力学研究表明Cu0.7Ta0.3/TiO2催化剂的反应遵循Langmuir-Hinshelwood机理,原位漫反射红外傅立叶变换光谱(in situ DRIFTS)验证了Ta的引入诱导了中间产物碳酸氢盐的生成,增加了催化剂中Cu+对CO的吸附能力,促进了表面吸附CO与氧的反应,从而提高了CO的氧化活性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
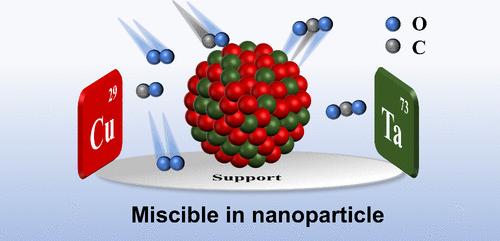
Enhancing the CO Oxidation Performance of Copper by Alloying with Immiscible Tantalum
Copper–tantalum (Cu–Ta) immiscible alloy nanoparticles (NPs) have been the subject of extensive research in the field of structural materials, due to their exceptional nanostructural stability and high-temperature creep properties. However, Cu is also a highly active oxidation catalyst due to its abundant valence changes. In this study, we have for the first time obtained homogeneous CuxTa1–x (x = 0.5, 0.7, 0.9, 1) nanoparticles by wet coreduction with an average particle size of approximately 30 nm. Testing verified all the CuxTa1–x/TiO2 (x = 0.5, 0.7, 0.9) showed higher CO oxidation activity than Cu/TiO2, with Cu0.7Ta0.3/TiO2 exhibiting the most promising performance. The temperature-programmed reduction with hydrogen demonstrated that Cu0.7Ta0.3/TiO2 exhibits enhanced redox properties. While kinetic studies indicated that the reaction of the Cu0.7Ta0.3/TiO2 catalyst followed the Langmuir–Hinshelwood mechanism, in situ diffuse reflectance infrared Fourier transform spectroscopy (in situ DRIFTS) verified the introduction of Ta induced the generation of bicarbonate as an intermediate product and increased the adsorption capacity of Cu+ on CO in the catalyst, which facilitated the reaction of surface adsorbed CO with oxygen and led to the enhanced CO oxidation activity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


