黄曲文B衍生物作为有效TRF2抑制剂治疗骨肉瘤的设计、合成和生物学评价
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
端粒重复结合因子2 (TRF2)是庇护蛋白复合体的重要组成部分,通常在骨肉瘤(OS)中过度表达,并与骨肉瘤的进展呈正相关。迄今为止,有效的体内应用TRF2抑制剂仍然有限。本研究设计并合成了一系列黄卡文B衍生物,并对其TRF2抑制和抗肿瘤活性进行了评价。其中活性化合物F2对U2OS和MG63细胞的TRF2表达有明显抑制作用,且具有较强的抗增殖活性,IC50值分别为5.28 μM和1.52 μM。此外,由于TRF2抑制,F2通过加速端粒缩短和丢失,显著抑制OS细胞增殖并诱导凋亡。机制上,F2通过直接结合TRF2TRFH结构域选择性抑制TRF2蛋白表达和端粒定位。此外,在mg63衍生的异种移植小鼠模型中,F2显示出强大的抗肿瘤功效和最小的毒性。这些发现表明F2是一种很有前景的骨肉瘤治疗药物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
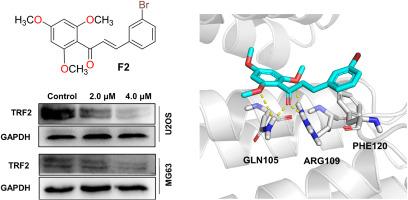
Design, synthesis, and biological evaluation of Flavokavain B derivatives as potent TRF2 inhibitors for the treatment of Osteosarcoma
Telomere repeat-binding factor 2 (TRF2) is a crucial component of the shelterin complex, commonly overexpressed in osteosarcoma (OS) and positively correlated with its progression. To date, effective TRF2 inhibitors for in vivo applications remain limited. In this study, a series of Flavokavain B derivatives were designed and synthesized, and their TRF2 inhibition and antitumor activity were evaluated. Among the tested compounds, the active compound F2 showed remarkable inhibition of TRF2 expression, along with potent antiproliferative activity in U2OS and MG63 cells, with IC50 values of 5.28 μM and 1.52 μM, respectively. Moreover, F2 significantly suppressed OS cell proliferation and induced apoptosis by accelerating telomere shortening and loss due to TRF2 inhibition. Mechanically, F2 selectively inhibited TRF2 protein expression and telomeric localization by directly binding to the TRF2TRFH domain. Furthermore, F2 demonstrated strong antitumor efficacy with minimal toxicity in an MG63-derived xenograft mouse model. These findings demonstrate that F2 is a promising drug candidate for the treatment of osteosarcoma.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


