人类DNPH1过渡态类似物揭示两种亲电迁移机制
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
DNPH1负责消除表观遗传修饰的核苷酸,5-羟甲基-2 ' -脱氧尿苷5 ' -单磷酸(hmdUMP),防止形成hmdUTP,一种诱导突变的核苷酸。DNPH1活性的丧失通过导致hmdUTP并入DNA而使PARP抑制抵抗的brca缺陷癌增敏。DNPH1水解hmdUMP的过程是通过Glu104和2-脱氧核糖5-磷酸之间的共价中间体进行的,然后是水解,这是一个有两个过渡态的反应循环。我们描述了DNPH1两个过渡态的过渡态模拟物的合成和表征。这两种过渡态都倾向于具有正电荷的缓蚀剂,为缓蚀剂的设计提供了基础。基态配合物显示亲核试剂的反应配位位于3.3-3.7 Å,而过渡态类似物将反应配位收紧,使亲核试剂位于2.7-2.8 Å。具有过渡态类似物的DNPH1的晶体结构揭示了亲电核位在离开基团和攻击亲核试剂之间迁移的过渡态。本文章由计算机程序翻译,如有差异,请以英文原文为准。
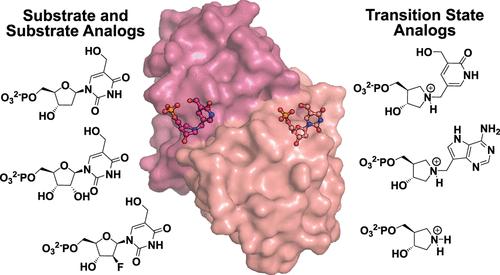
Transition State Analogs of Human DNPH1 Reveal Two Electrophile Migration Mechanisms
DNPH1 is responsible for eliminating the epigenetically modified nucleotide, 5-hydroxymethyl-2′-deoxyuridine 5′-monophosphate (hmdUMP), preventing formation of hmdUTP, a mutation-inducing nucleotide. Loss of DNPH1 activity sensitizes PARP inhibition-resistant BRCA-deficient cancers by causing incorporation of hmdUTP into DNA. Hydrolysis of hmdUMP by DNPH1 proceeds through a covalent intermediate between Glu104 and 2-deoxyribose 5-phosphate, followed by hydrolysis, a reaction cycle with two transition states. We describe synthesis and characterization of transition state mimics for both transition states of DNPH1. Both transition states prefer inhibitors with cationic charge at the anomeric center and provide a foundation for inhibitor design. Ground-state complexes show reaction coordinate nucleophiles poised 3.3–3.7 Å from the anomeric carbon while transition state analogs tighten the reaction coordinate to place the nucleophiles 2.7–2.8 Å from the anomeric carbon. Crystal structures of DNPH1 with transition state analogs reveal transition states where the electrophilic ribocation migrates between the leaving groups and attacking nucleophiles.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


