Pt/Al2O3催化剂上NO氧化的实验与动力学模型研究:共投丙烷和Pt粒度的影响
IF 4.3
2区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
摘要
Pt/Al2O3催化剂上NO氧化对优化SCR系统至关重要,但丙烷在不同Pt粒径催化剂上调节这一机制的作用尚不清楚。在这里,我们系统地研究了这一机制,以丙烷为代表的碳氢化合物。在小于2 nm的Pt颗粒上,丙烷有效地减少了反应过程中形成的PtOx,增强了NO的氧化。相反,在大于4 nm的Pt颗粒上,PtOx与丙烷的反应性较弱,并且保持不还原,阻碍NO氧化。根据这一发现,我们建立了一个考虑金属和氧化Pt活性位点的双位点动力学模型。该模型成功捕获了不同Pt尺寸的催化剂在丙烷存在下的NO氧化活性。这些发现有助于深入了解不同粒径DOC催化剂的催化行为,并有助于建立准确的动力学模型本文章由计算机程序翻译,如有差异,请以英文原文为准。
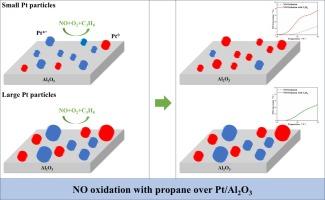
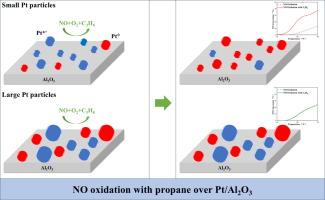
An experimental and kinetic modeling study of NO oxidation over Pt/Al2O3 catalysts: Effects of co-fed propane and Pt particle size
NO oxidation over Pt/Al2O3 catalysts is crucial for optimizing SCR systems, yet the role of propane in modulating this mechanism across catalysts with varying Pt particle sizes remains unclear. Herein, we systematically investigate this mechanism using propane as a representative hydrocarbon. On Pt particles less than 2 nm, propane effectively reduces PtOx formed during the reaction, enhancing NO oxidation. Conversely, on Pt particles larger than 4 nm, PtOx is less reactive with propane and remains unreduced, hindering NO oxidation. According to the findings, we developed a dual-site kinetic model accounting for both metallic and oxidized Pt active sites. This model successfully captures NO oxidation activity in the presence of propane across catalysts with different Pt sizes. These findings provide insights into the catalytic behavior of DOC catalysts with different grain sizes and aid in constructing accurate kinetic models.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Engineering Science
工程技术-工程:化工
CiteScore
7.50
自引率
8.50%
发文量
1025
审稿时长
50 days
期刊介绍:
Chemical engineering enables the transformation of natural resources and energy into useful products for society. It draws on and applies natural sciences, mathematics and economics, and has developed fundamental engineering science that underpins the discipline.
Chemical Engineering Science (CES) has been publishing papers on the fundamentals of chemical engineering since 1951. CES is the platform where the most significant advances in the discipline have ever since been published. Chemical Engineering Science has accompanied and sustained chemical engineering through its development into the vibrant and broad scientific discipline it is today.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


