小鼠全能卵裂球样细胞从合子基因组激活到着床后的胚胎发生模型
IF 19.8
1区 医学
Q1 CELL & TISSUE ENGINEERING
引用次数: 0
摘要
胚胎发育从合子基因组激活(ZGA)开始,最终产生胚泡进行着床。然而,体外系统模拟植入前发育仍然缺乏和具有挑战性。在这里,我们使用小鼠全能囊胚样细胞(TBLCs)分别发展自发分化和囊胚形成系统。我们发现Wnt信号可以促进tblc的快速扩增和培养基的优化。我们成功地建立了一个tblc自发分化系统,在该系统中,小鼠tblc (mtblc)首先转化为两种类型的zga样细胞(zlc),以Zscan4的表达为特征。令人惊讶的是,Zscan4-,而不是Zscan4+, zlc进一步通过中间的4细胞和8细胞/桑葚胚阶段产生外胚层、原始内胚层和滋养外胚层谱系。值得注意的是,单个tblc经过膨胀、压实和极化,有效地产生囊胚样结构,甚至植入后的卵柱状结构。最后,我们建立了基于tblc的分化和胚胎样结构形成系统来模拟早期胚胎发育,为评估和理解全能性提供了标准。本文章由计算机程序翻译,如有差异,请以英文原文为准。
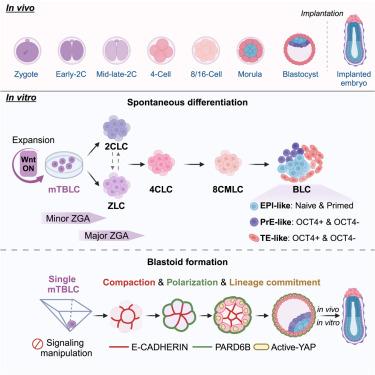
Mouse totipotent blastomere-like cells model embryogenesis from zygotic genome activation to post implantation
Embryo development begins with zygotic genome activation (ZGA), eventually generating blastocysts for implantation. However, in vitro systems modeling the pre-implantation development are still absent and challenging. Here, we used mouse totipotent blastomere-like cells (TBLCs) to develop spontaneous differentiation and blastoid formation systems, respectively. We found Wnt signaling enabled the rapid expansion of TBLCs and the optimization of their culture medium. We successfully developed a TBLC-spontaneous differentiation system in which mouse TBLCs (mTBLCs) firstly converted into two types of ZGA-like cells (ZLCs) distinguished by Zscan4 expression. Surprisingly, Zscan4-, but not Zscan4+, ZLCs further passed through intermediate 4-cell and then 8-cell/morula stages to produce epiblast, primitive endoderm, and trophectoderm lineages. Significantly, single TBLCs underwent expansion, compaction, and polarization to efficiently generate blastocyst-like structures and even post-implantation egg-cylinder-like structures. Conclusively, we established TBLC-based differentiation and embryo-like structure formation systems to model early embryonic development, offering criteria for evaluating and understanding totipotency.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell stem cell
生物-细胞生物学
CiteScore
37.10
自引率
2.50%
发文量
151
审稿时长
42 days
期刊介绍:
Cell Stem Cell is a comprehensive journal covering the entire spectrum of stem cell biology. It encompasses various topics, including embryonic stem cells, pluripotency, germline stem cells, tissue-specific stem cells, differentiation, epigenetics, genomics, cancer stem cells, stem cell niches, disease models, nuclear transfer technology, bioengineering, drug discovery, in vivo imaging, therapeutic applications, regenerative medicine, clinical insights, research policies, ethical considerations, and technical innovations. The journal welcomes studies from any model system providing insights into stem cell biology, with a focus on human stem cells. It publishes research reports of significant importance, along with review and analysis articles covering diverse aspects of stem cell research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


