(+)-3-(Z)- laurenatin和(+)-3-(Z)- isolaureatin不对称全合成中“孤对-孤对相互作用控制”异构化的计算见解
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
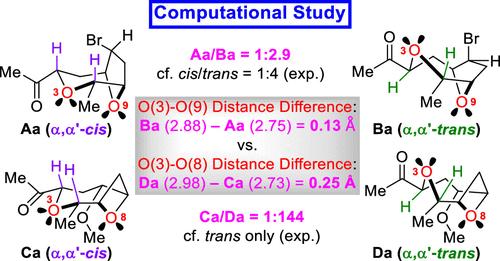
本文描述了我们的计算研究,以使我们之前在(+)-3-(Z)-异aureatin(1)和(+)-3-(Z)-laureatin(2)的全合成中报道的α,α ' -反式酮8和9的立体选择性外映合理化。密度泛函理论(DFT)使用适当截断的模型计算表明,α,α ' -反式酮比α,α ' -顺式酮更稳定。与实验结果非常吻合。计算结果表明,两环氧原子间原子间距离越长的异构体能量越低,说明两环氧原子间存在排斥相互作用。两个异构体之间的能量差异与溶剂极性相关,这一观察结果支持了这些依赖于距离的排斥性相互作用。最重要的是,与氧烷酮6相比,较大的氧间距离差可能导致氧烷酮7对外映体的立体选择性增强[0.25 Å(仅反式)vs 0.13 Å(反式/顺式= 4:1)]。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Computational Insights into “Lone Pair–Lone Pair Interaction-Controlled” Isomerization in the Asymmetric Total Syntheses of (+)-3-(Z)-Laureatin and (+)-3-(Z)-Isolaureatin
Described herein is our computational study to rationalize the stereoselective epimerization of α,α’-cis-disubstituted oxolane and oxetane ketones 6 and 7 to the corresponding α,α’-trans ketones 8 and 9 reported in our previous total syntheses of (+)-3-(Z)-isolaureatin (1) and (+)-3-(Z)-laureatin (2). Density functional theory (DFT) calculations using appropriately truncated models revealed that the α,α’-trans ketones are more stable than the α,α’-cis ketones, in very good agreement with experimental results. The computational results showed that the isomer with a longer interatomic distance between the two ring oxygen atoms was lower in energy, which suggested the presence of repulsive interactions between those oxygen atoms. Support for these distance-dependent repulsive interactions came from the observation that the energy differences between the two isomers correlated with the solvent polarity. Most importantly, a larger interoxygen distance difference could be responsible for the enhanced stereoselectivity for epimerization of oxetane ketone 7 compared to oxolane ketone 6 [0.25 Å (trans only) vs 0.13 Å (trans/cis = 4:1)].
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


