代谢拔河:微生物代谢形成对肠道病原体的定植抗性
IF 6.6
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
肠道微生物群的一个公认益处是,它能提供对肠道病原体的定植抵抗力。肠道微生物群及其产物可通过微生物与病原体之间的相互作用直接保护宿主免受入侵微生物的侵害,也可通过宿主与微生物群之间的相互作用间接保护宿主免受入侵微生物的侵害,从而调节免疫系统的功能。相比之下,肠道病原体已经进化出利用微生物群衍生代谢物的机制,以克服定植阻力并增加其致病潜力。本综述将重点介绍最近对代谢介导的定植阻力机制和肠道病原体用来克服这些阻力的毒力策略的研究,以及致病菌诱导炎症如何改变肠道景观并启用替代代谢途径。我们将重点关注肠道病原体如何抵消微生物群衍生代谢物的保护作用,以说明人们对代谢因素如何作为关键的毒力决定因素并克服定植阻力的认识不断提高。本文章由计算机程序翻译,如有差异,请以英文原文为准。

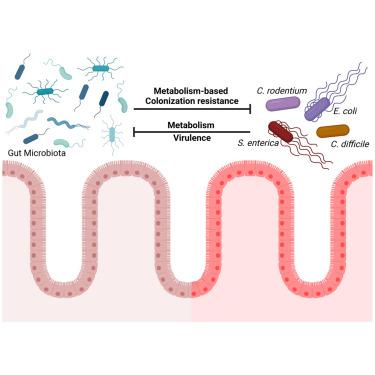
Metabolic tug-of-war: Microbial metabolism shapes colonization resistance against enteric pathogens
A widely recognized benefit of gut microbiota is that it provides colonization resistance against enteric pathogens. The gut microbiota and their products can protect the host from invading microbes directly via microbe-pathogen interactions and indirectly by host-microbiota interactions, which regulate immune system function. In contrast, enteric pathogens have evolved mechanisms to utilize microbiota-derived metabolites to overcome colonization resistance and increase their pathogenic potential. This review will focus on recent studies of metabolism-mediated mechanisms of colonization resistance and virulence strategies enteric pathogens use to overcome them, along with how induction of inflammation by pathogenic bacteria changes the landscape of the gut and enables alternative metabolic pathways. We will focus on how intestinal pathogens counteract the protective effects of microbiota-derived metabolites to illustrate the growing appreciation of how metabolic factors may serve as crucial virulence determinants and overcome colonization resistance.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell Chemical Biology
Biochemistry, Genetics and Molecular Biology-Molecular Medicine
CiteScore
14.70
自引率
2.30%
发文量
143
期刊介绍:
Cell Chemical Biology, a Cell Press journal established in 1994 as Chemistry & Biology, focuses on publishing crucial advances in chemical biology research with broad appeal to our diverse community, spanning basic scientists to clinicians. Pioneering investigations at the chemistry-biology interface, the journal fosters collaboration between these disciplines. We encourage submissions providing significant conceptual advancements of broad interest across chemical, biological, clinical, and related fields. Particularly sought are articles utilizing chemical tools to perturb, visualize, and measure biological systems, offering unique insights into molecular mechanisms, disease biology, and therapeutics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


