黑磷纳米片对多种抗生素耐药基因传播的激效效应
IF 12.2
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
黑磷纳米片(BPNS)的生产规模不断扩大,需求不断增加,不可避免地会导致环境泄漏。虽然 BPNSs 的生态毒理效应已经得到证实,但其间接的健康风险,如诱导病原菌耐药性增强,却往往被忽视。本研究探讨了 BPNSs 对携带多种抗性基因的 RP4 质粒促成的抗生素抗性基因(ARGs)水平基因转移的影响。结果表明,BPNSs 对细菌共轭基因转移表现出浓度依赖性的激素样效应。具体来说,在亚抑制浓度(0.0001-1 毫克/升)下,BPNSs 可促进属内和属间的共轭基因转移,表现出先上升后下降的现象,转移率分别上升了 1.5-3.1 倍和 1.5-3.3 倍。研究发现,BPNSs 能诱导活性氧(ROS)的产生,增加丙二醛的含量,并触发 SOS 反应,增强质粒的摄取。此外,BPNSs 还通过形成孔隙和上调外膜孔蛋白(OMPs)基因来增加膜的通透性。当 BPNSs 浓度较高(0.1-1 mg/L)时,由于细胞抗氧化系统受到破坏以及吸附过程发生变化,共轭频率受到抑制。这些发现强调了 BPNSs 对 ARGs 共轭转移的影响,补充了目前对与 BPNSs 相关的生物毒性和潜在生态风险的认识。本文章由计算机程序翻译,如有差异,请以英文原文为准。
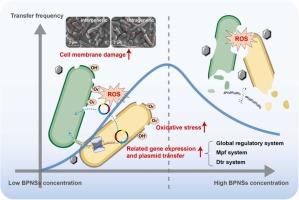
Hormesis-Like Effects of Black Phosphorus Nanosheets on the Spread of Multiple Antibiotic Resistance Genes
The production scalability and increasing demand for black phosphorus nanosheets (BPNSs) inevitably lead to environmental leakage. Although BPNSs' ecotoxicological effects have been demonstrated, their indirect health risks, such as inducing increased resistance in pathogenic bacteria, are often overlooked. This study explores the influence of BPNSs on the horizontal gene transfer of antibiotic resistance genes (ARGs) facilitated by the RP4 plasmid, which carries multiple resistance genes. The results indicated that BPNSs exhibited concentration-dependent hormesis-like effects on bacterial conjugation gene transfer. Specifically, at sub-inhibitory concentrations (0.0001-1 mg/L), BPNSs promoted both intra- and intergeneric conjugative transfer, demonstrating an initial increase followed by a decline, with transfer rates rising by 1.5-3.1-fold and 1.5-3.3-fold, respectively. BPNSs were found to induce reactive oxygen species (ROS) production, increase malondialdehyde levels, and trigger the SOS response, enhancing plasmid uptake. Additionally, BPNSs increased membrane permeability by forming pores and upregulating outer membrane porins (OMPs) genes. At higher BPNSs concentrations (0.1-1 mg/L), conjugative frequency was inhibited due to the disruption of the cellular antioxidant system and changes in the adsorption process. These findings underscore the influence of BPNSs on the conjugative transfer of ARGs, complementing current knowledge of the biotoxicity and potential ecological risks associated with BPNSs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


