离子印迹功能化壳聚糖增强和选择性吸附铀的双重策略——从酸性矿石渗滤液中快速高效回收
IF 13.2
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
从复杂废水中回收铀需要结合不同的工艺,包括从含有几种竞争金属离子的低浓度溶液中吸收金属。高效吸附剂(BTC/CH(s), 2-(苯并[d]噻唑-2-基)- n-氨基甲酰基乙酰胺接枝壳聚糖)的设计结合了高吸附能力和高选择性,采用双重策略:(a)选择有效的官能团(胺,酰胺,硫酯和羟基,在BTC/CH吸附剂中)和(b)调整反应基团的排列以适应配合物的特定形状(离子印迹IP与非离子印迹NIP材料)。采用这种双重策略设计了一种基于壳聚糖的吸附剂,该吸附剂具有高吸附量(≈1.5 mmol U g−1)、快速吸收(平衡:15-20 min)、卓越的稳定性(重复使用10次后性能损失有限)和强选择性(在等摩尔多组分溶液和预处理的酸性渗滤液中测试),在中等酸性pH(即4)下。离子模板策略有效地将选择性提高了5 - 10倍。吸附动力学用拟二级速率方程拟合,吸附等温线用Temkin方程模拟。吸附是放热的,自发的,离子模板可以达到更有组织的结构。该吸附剂对贱金属、碱金属和碱土金属具有较高的选择性,但对钍和稀土元素的分离效率较低。该吸附剂成功地用于回收酸性浸出液(Amberlite IRA-400和DOWEX 50分别用于回收U和稀土元素)和沉淀步骤(在pH 4下去除Al(III)/Fe(III))中的铀酰残留物。通过元素分析、FTIR和XPS光谱对吸附剂进行了表征,分析了材料的化学结构并确定了它们与U(VI)的相互作用。分析了结构性能和pHpzc值对吸附行为的支持作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
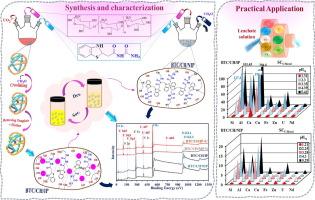
Dual strategy for enhanced and selective uranium sorption by ion-imprinting functionalized chitosan – Fast and efficient recovery from processed acid ore leachate
Uranium recovery from complex effluents requires the combination of different processes including metal sorption from low-concentration solutions containing several competitor metal ions. The design of efficient sorbents (BTC/CH(s), 2-(benzo[d]thiazol-2-yl)-N-carbamoyl acetamide grafted chitosan) that combine both high sorption capacity and high selectivity was achieved by adopting a dual strategy: (a) selecting efficient functional groups (amine, amide, thioester, and hydroxyl groups, in BTC/CH sorbents), and (b) adapting the arrangement of reactive groups appropriately to fit the specific shape of the complexes (ion-imprinting IP vs. non-ion-imprinted NIP materials). This dual strategy was applied to design a chitosan-based sorbent with high sorption capacity (≈1.5 mmol U g−1), fast uptake (equilibrium: 15–20 min), remarkable stability (limited loss of performances after 10 reuse cycles), and strong selectivity (tested both on equimolar multi-component solutions and pre-treated acid leachate), at moderately acidic pH (i.e., 4). Ion-templating strategy effectively improved selectivity by 5–10-folds. Uptake kinetics was fitted by the pseudo-second order rate equation, while the sorption isotherms were finely simulated by the Temkin equation. The sorption was exothermic, spontaneous, and the ion-templating allowed reaching more organized structure. The sorbent was highly selective against base metals, alkali and alkali-earth metals, but less efficient for the separation from thorium or rare-earth elements. The sorbent was successfully used for the recovery of uranyl residues from acidic leachates pre-treated with resins (Amberlite IRA-400 and DOWEX 50, for U and rare-earth element recovery, respectively) and precipitation step (removal of Al(III)/Fe(III) at pH 4). The sorbents were characterized by elemental analysis, FTIR and XPS spectroscopy for analyzing the chemical structure of the materials and identifying their interactions with U(VI). Textural properties and pHpzc values were analyzed for supporting sorption behaviors.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


