评估用于开发口服生物可用性 PROTAC 的脑龙导向弹头
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
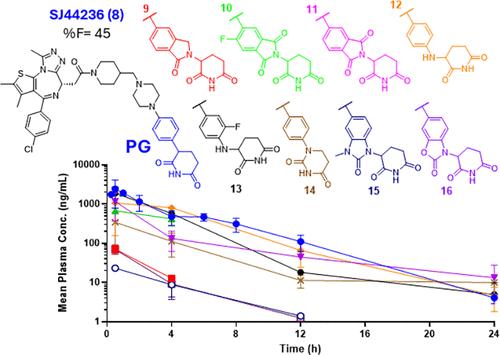
PROTACs通常占据经典类药物分子定义的物理化学空间之外,这在优化和开发口服给药方面往往面临相当大的挑战。我们之前报道了基于苯戊二胺(PG)的BET PROTAC SJ995973,与直接基于沙利度胺的类似物dBET1相比,具有更好的总体体外降解和抗增殖活性,但同样较差的体内药代动力学特征。为了进一步证明PG的实用性,我们在这里描述了优化工作,从而发现了一种口服生物可利用的BET-PROTAC SJ44236(8),并与含有替代crbn定向弹头的类似物进行了全面的体外/体内比较研究。我们的研究强调了在优化口服protac时考虑弹头修改的重要性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Evaluation of Cereblon-Directing Warheads for the Development of Orally Bioavailable PROTACs
PROTACs usually occupy physicochemical space outside the one defined by classical drug-like molecules, which often presents considerable challenges in their optimization and development for oral administration. We have previously reported phenyl glutarimide (PG)-based BET PROTAC SJ995973, with improved overall in vitro degradation and antiproliferative activities compared to its direct thalidomide-based analogue dBET1, but similarly poor in vivo pharmacokinetic profile. To further demonstrate the PG utility, we describe here optimization efforts that led to the discovery of an orally bioavailable BET-PROTAC SJ44236 (8), and results of a comprehensive in vitro/vivo comparative study with analogues containing alternative CRBN-directing warheads. Our study highlights the importance of considering warhead modifications when optimizing PROTACs for oral delivery.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


