通过PIWI Argonaute分析RNA切割的结构
IF 48.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
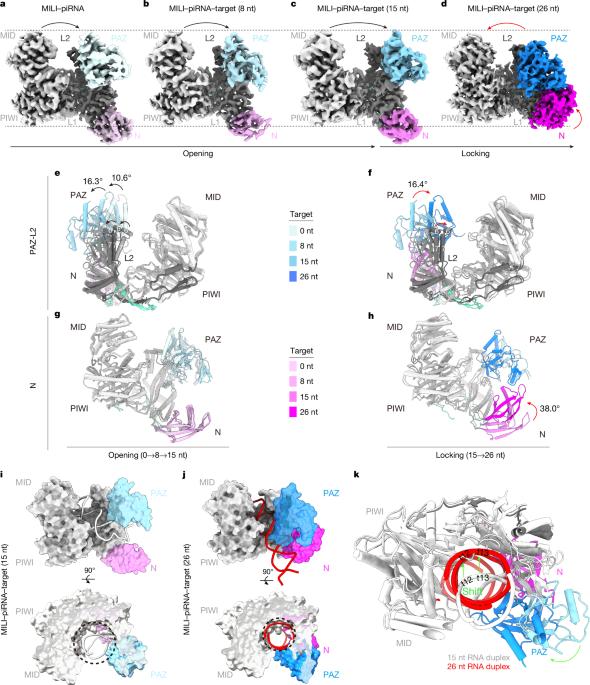
Argonaute蛋白分为AGO和PIWI两个分支。在大多数动物物种中,ago进化支蛋白在各种细胞类型中广泛表达,并调节正常基因表达1。相比之下,piwi进化枝蛋白在配子发生过程中主要抑制转座子并确保生育1,2。这两个分支通过碱基配对使用核酸向导来识别靶标,碱基配对对于启动靶标沉默至关重要,通常通过直接切割。ago进化支蛋白使用狭窄的通道来确保紧密的导向-靶标相互作用3。相比之下,PIWI蛋白具有更宽的通道,在配对过程中可能会出现错配,从而扩大了靶标沉默能力4,5。然而,piwi介导的靶标切割机制尚不清楚。本研究表明,在与靶标结合后,PIWI蛋白经历了从“开放”状态到“锁定”状态的构象变化,促进了碱基配对,提高了靶标切割效率。这种转变包括结合通道的变窄和piwi相互作用rna -靶标双工向piwi中瓣的重新定位,为双工稳定建立了广泛的接触。在这一转变过程中,我们还发现了一个中间的“逗号形”构象,它可能招募GTSF1,一种已知的增强PIWI切割活性的辅助蛋白6。GTSF1通过将PIWI结构域连接到RNA双工来促进向锁定状态的过渡,从而加速对有效靶向切割至关重要的构象变化。这些发现解释了PIWI- PIWI相互作用的RNA复合物在靶RNA切割中的分子机制,为PIWI蛋白的动态构象变化如何协调辅因子以保护配子发生提供了见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Structural insights into RNA cleavage by PIWI Argonaute
Argonaute proteins are categorized into AGO and PIWI clades. Across most animal species, AGO-clade proteins are widely expressed in various cell types, and regulate normal gene expression1. By contrast, PIWI-clade proteins predominantly function during gametogenesis to suppress transposons and ensure fertility1,2. Both clades use nucleic acid guides for target recognition by means of base pairing, crucial for initiating target silencing, often through direct cleavage. AGO-clade proteins use a narrow channel to secure a tight guide–target interaction3. By contrast, PIWI proteins feature a wider channel that potentially allows mismatches during pairing, broadening target silencing capability4,5. However, the mechanism of PIWI-mediated target cleavage remains unclear. Here we demonstrate that after target binding, PIWI proteins undergo a conformational change from an ‘open’ state to a ‘locked’ state, facilitating base pairing and enhancing target cleavage efficiency. This transition involves narrowing of the binding channel and repositioning of the PIWI-interacting RNA–target duplex towards the MID-PIWI lobe, establishing extensive contacts for duplex stabilization. During this transition, we also identify an intermediate ‘comma-shaped’ conformation, which might recruit GTSF1, a known auxiliary protein that enhances PIWI cleavage activity6. GTSF1 facilitates the transition to the locked state by linking the PIWI domain to the RNA duplex, thereby expediting the conformational change critical for efficient target cleavage. These findings explain the molecular mechanisms underlying PIWI–PIWI-interacting RNA complex function in target RNA cleavage, providing insights into how dynamic conformational changes from PIWI proteins coordinate cofactors to safeguard gametogenesis. After target binding, PIWI proteins undergo a conformational change from ‘open’ to ‘locked’, facilitating base pairing and enhancing target cleavage efficiency, providing insights into how dynamic conformational changes from PIWI coordinate cofactors to safeguard gametogenesis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


