从共价到非共价:金属在激活配体位点实现非共价相互作用(NCIs)中的作用
IF 20.3
1区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
本文综述了在配位和超分子化学交叉领域的一个新兴化学领域:金属诱导的非共价相互作用配体位点的增强(NCIs)。这个新领域探讨了金属配位如何放大配体参与各种NCIs的能力,包括卤素、硫、烟原和四元键,以及π-空穴相互作用。最近的研究揭示了两种不同类型的活化:高氧化态金属的亲电增强和低氧化态金属的罕见亲核活化。这两种机制通常都能显著提高NCI结合能,甚至可以在配体上产生新的非共价结合位点。一个值得注意的发现是铜(I)诱导的异氰化物碳的亲核活化卤素键,而不是更常见的亲电活化。金属诱导的增强和非共价结合配体位点的创建为设计固体含金属系统提供了创新策略,可能影响晶体工程和材料科学等多个领域。本文章由计算机程序翻译,如有差异,请以英文原文为准。

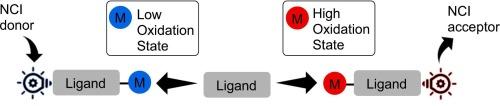
From covalent to noncovalent: The role of metals in activating ligand sites toward noncovalent interactions (NCIs)
This review highlights an emerging area in chemistry at the intersection of coordination and supramolecular chemistry: the metal-induced enhancement of ligand sites for noncovalent interactions (NCIs). This novel field explores how metal coordination amplifies ligands' capacity to engage in various NCIs, including halogen, chalcogen, pnictogen, and tetrel bonding, as well as π-hole interactions. Recent studies reveal two distinct types of activation: electrophilic enhancement by high-oxidation state metals, and the rarer nucleophilic activation by low-oxidation state metals. Both mechanisms often increase NCI binding energies significantly and can even generate new noncovalent binding sites on ligands. A notable discovery is the copper(I)-induced nucleophilic activation of isocyanide carbons for halogen bonding, contrasting with the more common electrophilic activation. The metal-induced enhancement and creation of ligand sites for noncovalent binding offers innovative strategies for designing solid metal-containing systems, potentially impacting diverse areas such as crystal engineering and materials science.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Coordination Chemistry Reviews
化学-无机化学与核化学
CiteScore
34.30
自引率
5.30%
发文量
457
审稿时长
54 days
期刊介绍:
Coordination Chemistry Reviews offers rapid publication of review articles on current and significant topics in coordination chemistry, encompassing organometallic, supramolecular, theoretical, and bioinorganic chemistry. It also covers catalysis, materials chemistry, and metal-organic frameworks from a coordination chemistry perspective. Reviews summarize recent developments or discuss specific techniques, welcoming contributions from both established and emerging researchers.
The journal releases special issues on timely subjects, including those featuring contributions from specific regions or conferences. Occasional full-length book articles are also featured. Additionally, special volumes cover annual reviews of main group chemistry, transition metal group chemistry, and organometallic chemistry. These comprehensive reviews are vital resources for those engaged in coordination chemistry, further establishing Coordination Chemistry Reviews as a hub for insightful surveys in inorganic and physical inorganic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


