具有快速固硫反应的全固态锂-S 电池
IF 48.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
凭借高比能、高安全性和低成本的承诺,全固态锂硫电池(ASSLSB)是下一代储能的理想选择1,2,3,4,5。但由于固-固硫氧化还原反应(SSSRR)在三相边界处缓慢而导致的速率性能差和循环寿命短的问题仍有待解决。在这里,我们展示了一种由硫代磷酸碘化锂(LBPSI)玻璃相固体电解质(GSEs)实现的快速SSSRR。基于I−和I2/I3−之间的可逆氧化还原,固体电解质(SE) -以及作为超离子导体-作为表面氧化还原介质,促进了固-固两相边界的缓慢反应,从而大大增加了活性位点的密度。通过这种机制,ASSLSB表现出超快充电能力,在2C(30°C)充电时显示出1,497 mAh g−1硫的高比容量,而在20C时仍然保持784 mAh g−1硫。值得注意的是,在60°C下以150℃的极端倍率充电时,可实现432 mAh g−1硫的比容量。此外,该电池在5C(25°C)下具有超过25,000次循环的优异稳定性,容量保持率为80.2%。我们期望我们在氧化还原介导的SSSRR上的工作将为开发高能量和安全的先进ASSLSBs铺平道路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
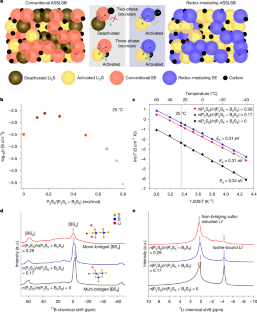
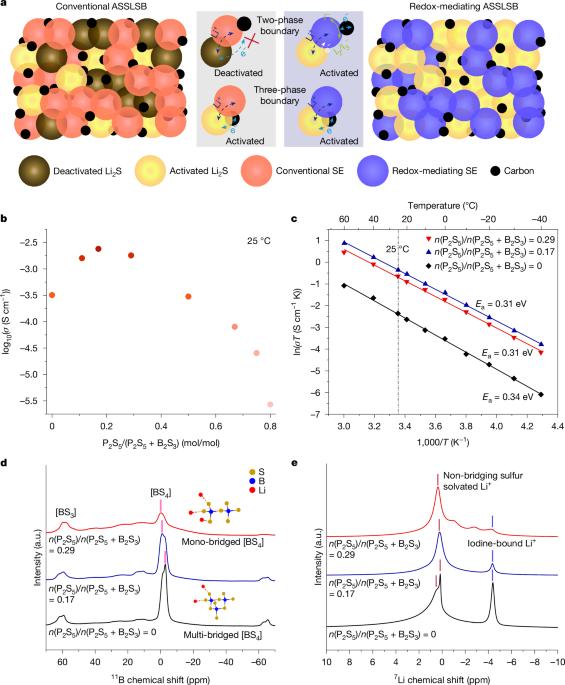
All-solid-state Li–S batteries with fast solid–solid sulfur reaction
With promises for high specific energy, high safety and low cost, the all-solid-state lithium–sulfur battery (ASSLSB) is ideal for next-generation energy storage1–5. However, the poor rate performance and short cycle life caused by the sluggish solid–solid sulfur redox reaction (SSSRR) at the three-phase boundaries remain to be solved. Here we demonstrate a fast SSSRR enabled by lithium thioborophosphate iodide (LBPSI) glass-phase solid electrolytes (GSEs). On the basis of the reversible redox between I− and I2/I3−, the solid electrolyte (SE)—as well as serving as a superionic conductor—functions as a surficial redox mediator that facilitates the sluggish reactions at the solid–solid two-phase boundaries, thereby substantially increasing the density of active sites. Through this mechanism, the ASSLSB exhibits ultrafast charging capability, showing a high specific capacity of 1,497 mAh g−1sulfur on charging at 2C (30 °C), while still maintaining 784 mAh g−1sulfur at 20C. Notably, a specific capacity of 432 mAh g−1sulfur is achieved on charging at an extreme rate of 150C at 60 °C. Furthermore, the cell demonstrates superior cycling stability over 25,000 cycles with 80.2% capacity retention at 5C (25 °C). We expect that our work on redox-mediated SSSRR will pave the way for developing advanced ASSLSBs that are high energy and safe. By using lithium thioborophosphate iodide glass-phase solid electrolytes in all-solid-state lithium–sulfur batteries, fast solid–solid sulfur redox reaction is demonstrated, leading to cells with ultrafast charging capability, superior cycling stability and high capacity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


