通过人工分子马达催化传递化学能的
IF 48.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
细胞表现出一系列由动力蛋白通过催化作用产生的机械活动。这就提出了一个基本的问题,即化学反应的加速如何使反应释放的能量被分子催化剂2、3、4、5、6、7转导(从而做功)。在这里,我们展示了化学能转化为机械力的分子水平转导,其形式是交联聚合物凝胶在人工催化驱动的分子马达的定向旋转驱动下的动力收缩和动力再膨胀。转子围绕催化驱动电机的定子连续360°旋转,凝胶聚合物框架中的分子使交联网络的聚合物链相互缠绕。这逐渐增加了扭曲和收紧缠结,导致凝胶宏观收缩到其原始体积的大约70%。随后加入相反的对映体燃料系统,使马达分子向相反方向旋转,解开缠结,使凝胶重新膨胀。在新的方向上持续的强力扭转会导致凝胶重新收缩。除了驱动外,凝胶中的马达分子旋转还会产生其他化学和物理结果,包括杨氏模量和存储模量的变化,后者与马达旋转引起的链交叉的增加成正比。合成有机催化剂抗负载的实验演示及其能量转导机制,为围绕生物马达产生力的机制和人工分子纳米技术的设计原则6,10,11,12,13,14的争论提供了信息。本文章由计算机程序翻译,如有差异,请以英文原文为准。
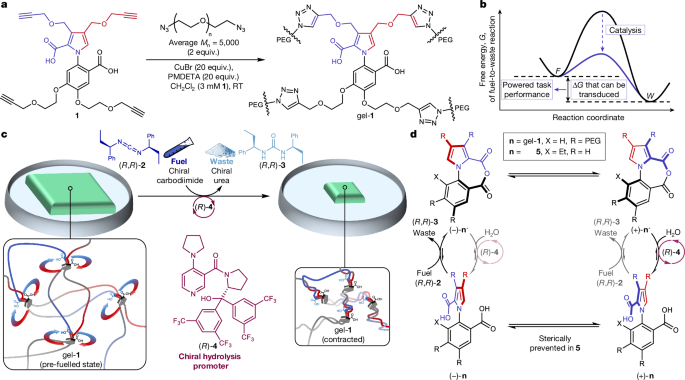
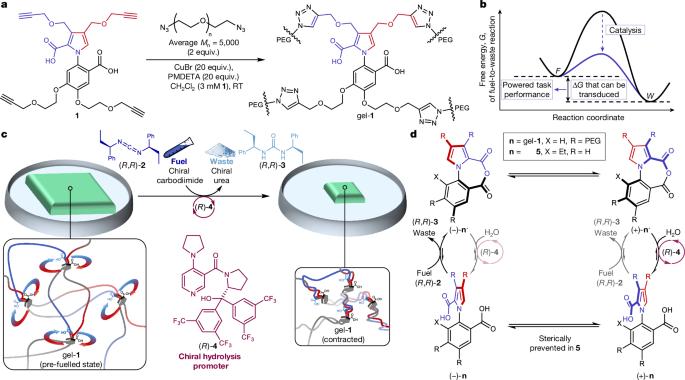
Transducing chemical energy through catalysis by an artificial molecular motor
Cells display a range of mechanical activities generated by motor proteins powered through catalysis1. This raises the fundamental question of how the acceleration of a chemical reaction can enable the energy released from that reaction to be transduced (and, consequently, work to be done) by a molecular catalyst2–7. Here we demonstrate the molecular-level transduction of chemical energy to mechanical force8 in the form of the powered contraction and powered re-expansion of a cross-linked polymer gel driven by the directional rotation of artificial catalysis-driven9 molecular motors. Continuous 360° rotation of the rotor about the stator of the catalysis-driven motor-molecules incorporated in the polymeric framework of the gel twists the polymer chains of the cross-linked network around one another. This progressively increases writhe and tightens entanglements, causing a macroscopic contraction of the gel to approximately 70% of its original volume. The subsequent addition of the opposite enantiomer fuelling system powers the rotation of the motor-molecules in the reverse direction, unwinding the entanglements and causing the gel to re-expand. Continued powered twisting of the strands in the new direction causes the gel to re-contract. In addition to actuation, motor-molecule rotation in the gel produces other chemical and physical outcomes, including changes in the Young modulus and storage modulus—the latter is proportional to the increase in strand crossings resulting from motor rotation. The experimental demonstration of work against a load by a synthetic organocatalyst, and its mechanism of energy transduction6, informs both the debate3,5,7 surrounding the mechanism of force generation by biological motors and the design principles6,10–14 for artificial molecular nanotechnology. A cross-linked polymer gel driven by artificial molecular motors transforms chemical energy into mechanical force, achieving powered contraction and re-expansion, demonstrating a considerable advance in understanding energy transduction mechanisms and informing nanotechnology design principles.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


