元素冷冻成像显示sos1依赖性空泡钠积累
IF 48.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
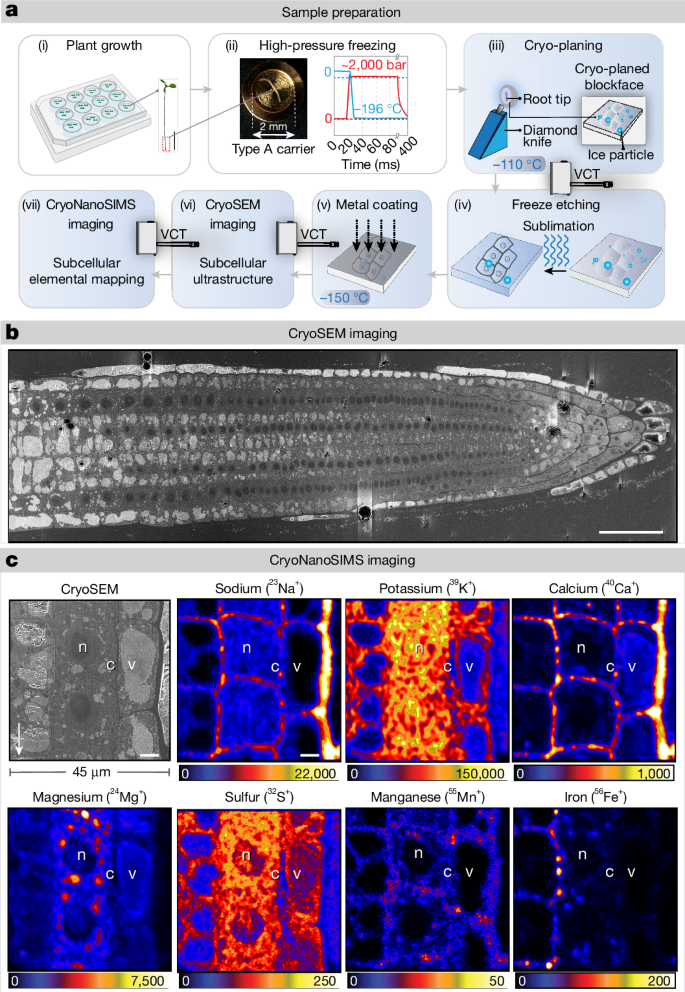
在全球范围内,土壤盐度的不断增加给农作物造成了重大损失;因此,了解植物对盐(钠)胁迫的反应非常重要。植物通过涉及高度元素相互依存的复杂过程进行亚细胞区隔,从而避免钠的毒性。目前可视化钠与其他元素的技术要么是间接的,要么缺乏分辨率。在这里,我们使用了新开发的低温纳米级二次离子质谱离子微探针1,它可以对低温保存的样品进行高分辨率元素成像,并揭示拟南芥和水稻根分生组织细胞中关键宏量营养元素和微量营养元素的亚细胞分布。我们发现钠的分布发生了意想不到的浓度依赖性变化,在外部钠浓度较低时,钠在细胞壁中积累,而在钠浓度较高时,钠在液泡中积累。我们的结论是,在根分生组织中,NHX 家族钠/质子拮抗剂 SALT OVERLY SENSITIVE 1(又称 Na+/H+ 交换子 7;SOS1/NHX7)的一个关键功能是将钠封闭在液泡中,而不是将钠挤出到细胞外空间。使用新的基因组互补荧光标记 SOS1 变体证实了这一点。我们发现,除质膜外,SOS1 还会在晚期内膜/前液泡以及液泡中大量积聚,这支持了 SOS1 在液泡钠螯合中的作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Elemental cryo-imaging reveals SOS1-dependent vacuolar sodium accumulation
Increasing soil salinity causes significant crop losses globally; therefore, understanding plant responses to salt (sodium) stress is of high importance. Plants avoid sodium toxicity through subcellular compartmentation by intricate processes involving a high level of elemental interdependence. Current technologies to visualize sodium, in particular, together with other elements, are either indirect or lack in resolution. Here we used the newly developed cryo nanoscale secondary ion mass spectrometry ion microprobe1, which allows high-resolution elemental imaging of cryo-preserved samples and reveals the subcellular distributions of key macronutrients and micronutrients in root meristem cells of Arabidopsis and rice. We found an unexpected, concentration-dependent change in sodium distribution, switching from sodium accumulation in the cell walls at low external sodium concentrations to vacuolar accumulation at stressful concentrations. We conclude that, in root meristems, a key function of the NHX family sodium/proton antiporter SALT OVERLY SENSITIVE 1 (also known as Na+/H+ exchanger 7; SOS1/NHX7) is to sequester sodium into vacuoles, rather than extrusion of sodium into the extracellular space. This is corroborated by the use of new genomic, complementing fluorescently tagged SOS1 variants. We show that, in addition to the plasma membrane, SOS1 strongly accumulates at late endosome/prevacuoles as well as vacuoles, supporting a role of SOS1 in vacuolar sodium sequestration. This study demonstrates that cryo nanoscale secondary ion mass spectrometry (CryoNanoSIMS) enables direct multi-elemental imaging at subcellular resolution of macro- and micronutrients or trace elements in plants and may provide insights into the in vivo roles of many transporters.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


