用于高效电催化硝酸盐还原成氨的碳点增效活性氢
IF 6.3
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
电催化硝酸还原反应(NO3RR)作为一种将环境废弃物转化为肥料的手段受到了广泛的关注。然而,NO3RR的活性和效率受到与硝酸(NO3-)加氢竞争的活性氢(H ads)不可避免的自偶联的严重阻碍。本研究通过将多功能碳点(CDs)与硼铁矿(Cu5FeS4)结合,开发了一种高效的电催化剂,以全面控制氢离子的吸附和转化。结果表明,CDs/Cu5FeS4的NH3产率为215.19 μmol h-1 cm-2,法拉第效率为86.52%。重要的是,与纯Cu5FeS4相比,CDs的引入可以大大提高NH3产率和FE,即使在很宽的NO3-浓度范围内也是如此。同时,该催化剂通过流动池成功地将模拟地下水NO3转化为NH3。循环伏安法和电子顺磁共振实验表明,CDs有效地促进了氢离子的生成,并参与了NO3-氢化反应。动力学同位素实验揭示了CDs在加速水解离和质子转移过程中的作用。原位拉曼光谱分析表明,CDs能有效促进NO3-和NO2-对NH3的吸附和转化。DFT计算表明,CDs显著降低了关键速率决定步骤(NO3到NO2)的能垒,从而促进了ads与含n中间体之间的加氢反应。这项工作为利用CDs促进H - ads的生成和转化来提高氨的催化活性提供了一个有趣的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
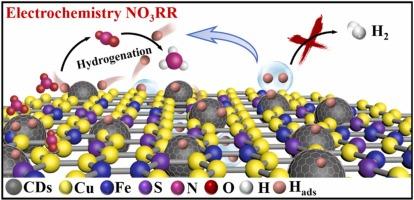
Carbon dots-boosted active hydrogen for efficient electrocatalytic reduction of nitrate to ammonia
Electrocatalytic nitrate reduction reaction (NO3RR) has gained great attention as a means of transforming environmental waste into fertilizers. However, the activity and efficiency of NO3RR is severely hindered by the inevitable self-coupling of active hydrogen (H*ads) competing with nitrate (NO3-) hydrogenation. Here, an efficient electrocatalyst is developed by combining multifunction carbon dots (CDs) with bornite (Cu5FeS4) towards the comprehensive manipulation of the adsorption and conversion of H*ads. As a result, the optimized CDs/Cu5FeS4 presents a remarkable NH3 yield of 215.19 μmol h−1 cm−2 with a Faradaic efficiency of 86.52 %. Importantly, the introduction of CDs can greatly increase the NH3 yield and FE compared with pure Cu5FeS4, even under a wide range of NO3- concentrations. Meanwhile, the catalyst can successfully transform simulated NO3- groundwater into NH3 by using a flow cell. The cyclic voltammetry and electron paramagnetic resonance tests indicate that CDs effectively promote the production of H*ads and participate in NO3- hydrogenation. Kinetic isotope experiments reveal the role of CDs in accelerating water dissociation and proton transfer processes. In-situ Raman spectroscopy demonstrate that CDs effectively boosts the adsorption and conversion of NO3- to NO2- and NO2- to NH3. And DFT calculations reveal CDs significantly reduce the energy barrier of the key rate-determining step (*NO3 to *NO2), which thus promotes the hydrogenation reaction between H*ads and N-containing intermediates. This work provides an interesting insight for enhancing the catalytic activity of ammonia by utilizing CDs to promote the generation and conversion of H*ads.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Alloys and Compounds
工程技术-材料科学:综合
CiteScore
11.10
自引率
14.50%
发文量
5146
审稿时长
67 days
期刊介绍:
The Journal of Alloys and Compounds is intended to serve as an international medium for the publication of work on solid materials comprising compounds as well as alloys. Its great strength lies in the diversity of discipline which it encompasses, drawing together results from materials science, solid-state chemistry and physics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


