提高过氧单硫酸盐类fenton反应的阳极性能:磷掺杂的作用
IF 13.2
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
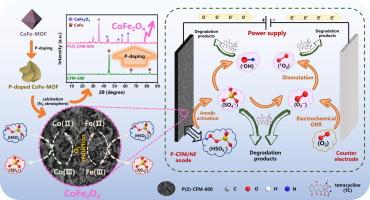
由于磷(P)掺杂能够提高过渡金属材料的催化活性,因此备受关注。然而,在基于过一硫酸盐(PMS)的电化学芬顿样反应(EFR)中应用这种策略的可行性和机理仍不清楚。在此,我们以掺杂 P 的双金属(Co/Fe)金属有机框架(MOF)和泡沫镍(NF)为前驱体和基底,开发了一种新型阳极(P-CFM/NF)。在最佳条件下,掺杂 P 显著提高了阳极 PMS 的活化性能,在 3 到 11 的宽 pH 值范围内,60 分钟内污染物去除率几乎达到 100%。进一步的研究表明,掺杂 P 促进了高活性 CoFe2O4 的形成,将电子转移电阻(Rct)降低了 5.27 倍,并降低了蓄势阳极电位,从而提高了基于 PMS 的 EFR 的阳极性能。淬火实验和电子顺磁共振(EPR)测试表明,P-CFM/NF 阳极在 PMS 活化过程中产生的主要反应物有 SO4-、OH-、O2-和 1O2。此外,阴极氧还原反应(ORR)为 O2--的生成提供了另一条途径,而 O2--是 1O2 生成不可或缺的前体。这项研究介绍了一种有效的方法,即利用掺杂 P 的策略来提高双金属 MOF 衍生材料在类似芬顿反应中的催化活性。此外,它还为通过基于 PMS 的 EFR 技术促进有机污染物降解提供了宝贵的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Enhancement of anodic performance toward peroxymonosulfate-based fenton-like reactions: Role of phosphorus-doping
Phosphorus (P)-doping has gained significant attention due to its ability to enhance the catalytic activity of transition metal materials. However, the feasibility and mechanism of applying this strategy in peroxymonosulfate (PMS)-based electrochemical Fenton-like reactions (EFR) remain unclear. Herein, a novel anode (P-CFM/NF) was developed using a P-doped bimetallic (Co/Fe) metal–organic framework (MOF) and nickel foam (NF) as the precursor and substrate, respectively. Under optimal conditions, P-doping significantly improved the anodic PMS activation performance, with pollutant removal efficiency reaching almost 100 % within 60 min across a wide pH range of 3 to 11. Further investigations showed that P-doping facilitated the formation of highly reactive CoFe2O4, reduced electron transfer resistance (Rct) by a factor of 5.27, and lowered the poised anodic potential, thereby enhancing the anodic performance of PMS-based EFR. Quenching experiments and electron paramagnetic resonance (EPR) tests demonstrated that the main reactive species generated during the PMS activation process induced by P-CFM/NF anode were SO4•−, OH•, O2•− and 1O2. Additionally, the cathodic oxygen reduction reaction (ORR) provided another pathway for generation of O2•−, which served as an indispensable precursor for 1O2 formation. This study introduced an effective approach utilizing a P-doping strategy to enhance the catalytic activity of bimetallic MOF-derived materials in Fenton-like reactions. Furthermore, it also offers valuable insights into the advancement of organic pollutant degradation through PMS-based EFR.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


