富氧氧化锌吸附/光催化降解动力学与阳离子/阴离子染料表面官能团的关系
IF 13.2
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
本研究探讨了染料电荷和官能团(如吸电子基团和供电子基团)对四种目标染料(即两种阳离子染料(孔雀石绿和红花红O)和两种阴离子染料(甲基红和玫瑰红)的吸附和光催化降解(PCD)的影响,并利用富氧空位氧化锌作为催化剂。在500 °C下,使用10 % H2/Ar气体混合物进行氢还原,引入氧空位。建立了一个综合的动力学模型,该模型考虑了可逆的吸附-脱附过程,并区分了羟基自由基(在溶液中)和电子空穴(在催化剂表面)的降解。此外,该模型结合了染料的形态(基于它们的pKa值)来解释ph依赖的吸附行为。结果表明,在pH为3时,所有染料均处于完全质子化状态,吸附去除率最大。密度泛函理论(DFT)计算也可以生成静电电位(ESP)图,表明在酸性条件下,大多数PCD是如何在催化剂表面发生的,而在碱性条件下,PCD是如何在散装溶液中发生的。因此,具有供电子基团的阳离子染料在pH为11时表现出最高的降解率,因为它们在碱性条件下容易与羟基自由基反应。相反,具有吸电子基团的阴离子染料在pH为3时达到最大降解率,此时它们优先与催化剂表面的电子空穴发生反应。最后,量子产率计算表明,阳离子染料在pH值为11时的最大QY为7.12 × 10−5,阴离子染料在pH值为3时的最大QY为3.86 × 10−5。本文章由计算机程序翻译,如有差异,请以英文原文为准。
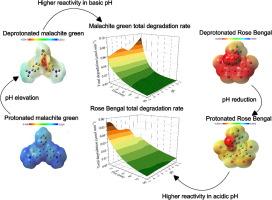
The adsorption/photocatalytic degradation kinetics of oxygen vacancy-enriched ZnO in relation to surface functional groups of cationic/anionic dyes
This study explores the effects of dye charge and functional groups (e.g., electron-withdrawing and electron-donating groups) on the adsorption and photocatalytic degradation (PCD) of all four target dyes (i.e., two cationic (malachite green and safranin O) vs. two anionic dyes (methyl red and rose bengal)) using oxygen vacancy-enriched ZnO as a catalyst. Oxygen vacancies are introduced by hydrogen reduction using a 10 % H2/Ar gas mixture at 500 °C. A comprehensive kinetic model has been developed which accounts for reversible adsorption–desorption processes and distinguishes between degradation by hydroxyl radicals (in the solution) and those by electron-holes (on the catalyst surface). Additionally, the model incorporates the speciation of dyes (based on their pKa values) to account for pH-dependent adsorption behaviors. The results indicate the maximum adsorption removal occurs at pH 3 where all dyes are at their fully protonated state. Density functional theory (DFT) calculations are also performed to generate electrostatic potential (ESP) maps indicating how at acidic conditions most PCD occurs on the catalyst surface while at basic pH occurs in the bulk solution. Accordingly, cationic dyes with electron-donating groups exhibit the highest degradation rates at pH 11, as they readily react with hydroxyl radicals in basic conditions. Conversely, anionic dyes with electron-withdrawing groups reach their maximum degradation rates at pH 3, where they preferentially react with electron-holes on the catalyst surface. Finally, the quantum yield calculations demonstrate that cationic dyes reach their maximum QY of 7.12 × 10−5 at pH 11 while anionic dyes achieve their highest QY of 3.86 × 10−5 at pH 3.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


