铜催化非活化烯烃的三组分氟烷基烷基化反应
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
电子非活化烯烃的对映选择性三组分二碳官能化仍然是一个重大的挑战。在温和的条件下,研究了铜催化的具有不同末端炔和氟烷基卤化物的非活化烯烃的高区域选择性和对映选择性氟烷基烷基化反应。除了氟烷基卤化物外,Togni的试剂也可以参与反应,递送具有高对映选择性的手性β-三氟甲基炔烃。该方法具有良好的官能团耐受性,促进了各种生物活性分子的后期衍生化。这种化学反应的成功是通过使用一个大体积的吲哚取代的BOPA配体实现的。DFT计算表明,自由基氟烷基烷基化是通过氟定向外球途径实现的。机理研究表明,酰胺基团是实现高立体选择性的关键,因为在酰胺上的氟烷基和Mes基团之间可以形成独占的F···H氢键,以稳定si自由基偶联过渡态。本文章由计算机程序翻译,如有差异,请以英文原文为准。
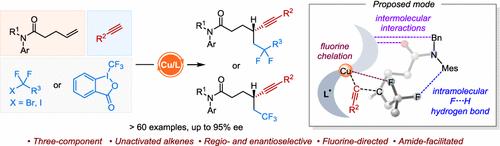
Copper-Catalyzed Enantioselective Three-Component Fluoroalkylalkynylation of Unactivated Alkenes
The enantioselective three-component dicarbonfunctionalization of electronically unactivated alkenes continues to pose a significant challenge. In this work, a copper-catalyzed highly regio- and enantioselective fluoroalkylalkynylation of unactivated alkenes with diverse terminal alkynes and fluoroalkyl halides under mild conditions is developed. In addition to fluoroalkyl halides, Togni’s reagent can also participate in the reaction, delivering chiral β-trifluoromethyl alkynes with high enantioselectivities. This method exhibits good functional group tolerance, facilitating the late-stage derivatization of a variety of biologically active molecules. The success of this chemistry was achieved by using a bulky indene-substituted BOPA ligand. DFT calculations indicate that the radical fluoroalkylalkynylation is achieved through a fluorine-directed outer-sphere pathway. Mechanistic studies reveal that the amide group is crucial for achieving high stereoselectivities because the exclusive F···H hydrogen bonding between the fluoroalkyl group and the Mes group on the amide can be formed to stabilize the Si-radical coupling transition state.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


