口服补充剂中的锌纳米颗粒在肾肿瘤中积聚并激发抗肿瘤免疫反应
IF 37.2
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
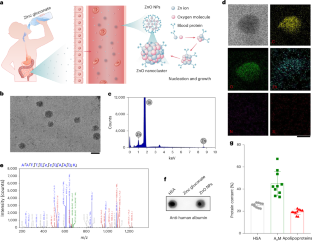
一个成功的治疗结果在实体瘤的治疗需要有效的肿瘤内药物积累和保留。在这里,我们证明了口服补品中的葡萄糖酸锌与血浆蛋白组装形成ZnO纳米颗粒,选择性地积聚在乳头状Caki-2肾肿瘤中,并促进树突状细胞和细胞毒性CD8+ T细胞向肿瘤组织募集。肾肿瘤靶向是通过锌离子与金属硫蛋白1x蛋白的优先结合介导的,金属硫蛋白1x蛋白在Caki-2肾肿瘤细胞中组成性过表达。这种结合事件进一步上调细胞内金属硫蛋白1x的表达,诱导额外的纳米颗粒结合和保留。在肿瘤动物模型和人类肾肿瘤样本中,我们发现氧化锌纳米颗粒主动穿过血管壁,实现肿瘤内的高蓄积。我们进一步探索了氧化锌纳米颗粒在小鼠和兔子癌症模型中传递化疗药物的这一特性。我们的研究结果表明,从补充剂中提取的ZnO纳米颗粒可以作为多功能药物传递和癌症免疫治疗平台。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Zinc nanoparticles from oral supplements accumulate in renal tumours and stimulate antitumour immune responses
A successful therapeutic outcome in the treatment of solid tumours requires efficient intratumoural drug accumulation and retention. Here we demonstrate that zinc gluconate in oral supplements assembles with plasma proteins to form ZnO nanoparticles that selectively accumulate into papillary Caki-2 renal tumours and promote the recruitment of dendritic cells and cytotoxic CD8+ T cells to tumour tissues. Renal tumour targeting is mediated by the preferential binding of zinc ions to metallothionein-1X proteins, which are constitutively overexpressed in Caki-2 renal tumour cells. This binding event further upregulates intracellular metallothionein-1X expression to induce additional nanoparticle binding and retention. In both tumour animal models and human renal tumour samples, we show that ZnO nanoparticles actively cross the vascular wall to achieve high intratumoural accumulation. We further explore this feature of ZnO nanoparticles for the delivery of chemotherapeutics to mouse and rabbit cancer models. Our findings demonstrate that ZnO nanoparticles derived from supplements can serve as a multifunctional drug delivery and cancer immunotherapy platform. Zinc gluconate in oral supplements associates with plasma proteins to form renal-tumour-accumulating ZnO nanoparticles, which have antitumoural immune activity and can also be used for the delivery of chemotherapeutic agents.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Materials
工程技术-材料科学:综合
CiteScore
62.20
自引率
0.70%
发文量
221
审稿时长
3.2 months
期刊介绍:
Nature Materials is a monthly multi-disciplinary journal aimed at bringing together cutting-edge research across the entire spectrum of materials science and engineering. It covers all applied and fundamental aspects of the synthesis/processing, structure/composition, properties, and performance of materials. The journal recognizes that materials research has an increasing impact on classical disciplines such as physics, chemistry, and biology.
Additionally, Nature Materials provides a forum for the development of a common identity among materials scientists and encourages interdisciplinary collaboration. It takes an integrated and balanced approach to all areas of materials research, fostering the exchange of ideas between scientists involved in different disciplines.
Nature Materials is an invaluable resource for scientists in academia and industry who are active in discovering and developing materials and materials-related concepts. It offers engaging and informative papers of exceptional significance and quality, with the aim of influencing the development of society in the future.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


