薄膜法快速定量测量纳米多孔材料中混合气体吸附平衡
IF 3.9
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
摘要
纳米多孔吸附材料是许多工业过程的关键部分,包括迅速发展的碳捕集工业。先进吸附剂的开发需要对吸附剂在混合气体条件下的性能进行评估。现有的测量技术往往是缓慢的、材料密集的,并且测量竞争性混合气体吸附的能力有限。我们开发了一种新技术,可以测量沉积在敏感微机电系统(MEMS)传感器上的吸附剂薄膜。该技术速度快,需要很少的材料,并且可以实时监测二元气体的吸附。我们报告了碳捕获MOF CALF-20在三种不同温度下对CO2/H2O混合气体等温线的测量。CALF-20中CO2/H2O混合物吸附的实测实验数据表明,理想吸附溶液理论(IAST)在提供组分负荷定量估计方面存在严重局限性。通过引入活度系数和使用实际吸附溶液理论(RAST)来量化与IAST的偏离。本文章由计算机程序翻译,如有差异,请以英文原文为准。
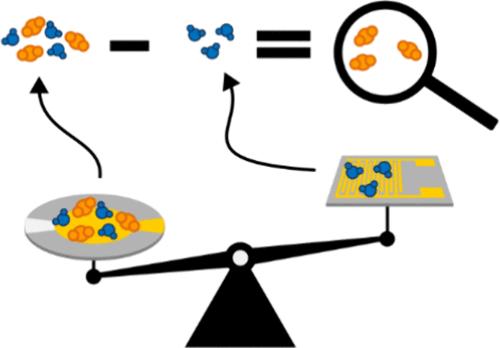
Thin-Film Approach to Rapid, Quantitative Measurements of Mixed-Gas Adsorption Equilibrium in Nanoporous Materials
Nanoporous adsorbent materials are a key part of many industrial processes, including the rapidly expanding carbon capture industry. The development of advanced sorbents requires an assessment of the sorbent’s performance under mixed-gas conditions. Existing measurement techniques tend to be slow, material-intensive, and have limited ability to measure competitive mixed-gas sorption. We have developed a novel technique that measures thin films of sorbents deposited onto sensitive microelectromechanical system (MEMS) transducers. This technique is fast, requires very little material, and enables real-time monitoring of binary gas sorption. We report measurements of CO2/H2O mixed-gas isotherms at three different temperatures on the carbon capture MOF CALF-20. The measured experimental data on CO2/H2O mixture adsorption in CALF-20 demonstrate the severe limitations of the Ideal Adsorbed Solution Theory (IAST) in providing a quantitative estimation of the component loadings. Departures from the IAST are quantified by the introduction of activity coefficients and the use of the Real Adsorbed Solution Theory (RAST).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


