g-CN纳米片修饰CuSe纳米粒子在超级电容器中的电化学研究
IF 6.3
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
世界能源危机的出现可以归结为工业化的快速发展。应该优先开发能够有效利用可再生能源的自主大容量储能技术。由于其优越的比电容(Cs)和灵活性,CuSe作为电极材料获得了极大的兴趣。然而,它的电化学功能受到缓慢离子转移和粒子聚集的阻碍。为此,利用g-CN等碳质材料,具有二维支撑、高氮浓度、大比表面积(SSA)等显著特性,可以通过减少团聚而显著提高电导率。在此,我们利用简单的水热方法在g-CN纳米片上制备了CuSe纳米颗粒。在相同电流密度(Cd)为1 a /g时,合成材料的最大Cs值为1109 F/g,明显大于纯CuSe (609 F/g)和g- cn (239 F/g),在5000次充放电循环后,其电容恢复率为97.6%。制备的材料具有较高的比能(SE = 54.82 Wh/kg)和比功率(SP = 645 W/kg)。此外,制备的CuSe/g- cn纳米复合材料具有更高的SSA (53 m2/g)和显著降低的电荷转移电阻(Rct, 0.76 Ω)。这些有利的结果提供了令人信服的证据,表明金属硒化物结合在g-CN上可以有效地用作超级电容器的替代电极。本文章由计算机程序翻译,如有差异,请以英文原文为准。
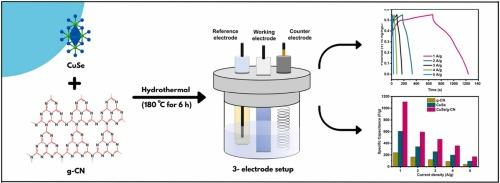
Electrochemical investigation of CuSe nanoparticles decorated over g-CN nanosheet for supercapacitor applications
The emergence of energy crisis in the world can be attributed to the rapid progression of industrialization. It is necessary to prioritize the development of autonomous high-capacity storage technologies that can effectively utilize renewable energy sources. CuSe has gained significant interest as an electrode material due to its superior specific capacitance (Cs) and flexibility. Nevertheless, its electrochemical functionality is hindered by slow ion transfers and the agglomeration of particles. For this purpose, the utilization of carbonaceous materials such as g-CN, which possess remarkable characteristics like 2D support, high nitrogen concentration and greater specific surface area (SSA), can significantly enhance electrical conductivity by reducing aggregation. Herein, we developed CuSe nanoparticles on g-CN nanosheets utilizing a simple hydrothermal approach. The synhesized material displays a maximum Cs of 1109 F/g which is significantly larger than pure CuSe (609 F/g) and g-CN (239 F/g) at same current density (Cd) of 1 A/g and retians 97.6 % of capacitance after 5000 charge-discharge cycles. As-prepared material demonstrated high specific energy (SE = 54.82 Wh/kg) and specific power (SP = 645 W/kg), accordingly. Besides, the fabricated CuSe/g-CN nanocomposite shows greater SSA (53 m2/g) with significantly lower charge transfer resistance (Rct, 0.74 Ω). The favourable outcomes provide compelling evidence that metal selenides incorporated on g-CN can be efficiently employed as substitute electrodes in supercapacitors.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Alloys and Compounds
工程技术-材料科学:综合
CiteScore
11.10
自引率
14.50%
发文量
5146
审稿时长
67 days
期刊介绍:
The Journal of Alloys and Compounds is intended to serve as an international medium for the publication of work on solid materials comprising compounds as well as alloys. Its great strength lies in the diversity of discipline which it encompasses, drawing together results from materials science, solid-state chemistry and physics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


