超小铁没食子酸配位聚合物纳米颗粒清除活性氧和抑制牛头病诱导的阿尔茨海默病的炎症。
IF 12.9
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
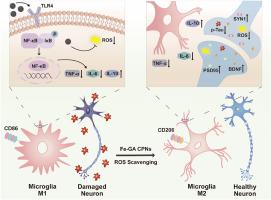
阿尔茨海默病(AD)是全球最常见的神经退行性疾病,目前尚无有效的治疗方法。阿尔茨海默病的一个重要病理标志是过度磷酸化的tau蛋白的积累,在阿尔茨海默病的进展中,活性氧(ROS)和神经炎症会使其恶化。因此,在许多研究中,减轻ROS和炎症已成为一种潜在的治疗策略。本文中,我们报道了由铁离子和没食子酸形成的超小配位聚合物纳米粒子(Fe-GA CPNs),它具有抗氧化和抗炎症的特性,可用于治疗AD。与没食子酸相比,制备的Fe-GA CPNs表现出明显的超氧化物歧化酶样、过氧化物酶样酶活性,并具有较好的水溶性去除ROS能力。我们证明了Fe-GA CPNs有效地缓解氧化应激,通过调节小胶质细胞极化向抗炎症表型改善炎症,并降低过度磷酸化的tau蛋白水平。此外,Fe-GA CPNs治疗可显著改善牛头病诱导的AD大鼠的认知功能,并对AD病理产生神经保护作用。这项研究强调了配位聚合物纳米颗粒作为治疗AD和其他tau相关神经退行性疾病的有希望的候选药物的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Ultrasmall iron-gallic acid coordination polymer nanoparticles for scavenging ROS and suppressing inflammation in tauopathy-induced Alzheimer's disease
Alzheimer's disease (AD) is the most prevalent neurodegenerative disorder globally, with no effective treatment available yet. A crucial pathological hallmark of AD is the accumulation of hyperphosphorylated tau protein, which is deteriorated by reactive oxygen species (ROS) and neuroinflammation in AD progression. Thus, alleviation of ROS and inflammation has become a potential therapeutic strategy in many studies. Herein, we reported ultrasmall coordination polymer nanoparticles formed by ferric ions and gallic acid (Fe-GA CPNs), which owned antioxidant and anti-inflammation properties for AD therapeutics. The facilely prepared Fe-GA CPNs exhibited remarkable superoxide dismutase-like, peroxidase-like enzyme activity, and ROS eliminating ability with great water solubility, compared with gallic acid. We demonstrated that Fe-GA CPNs effectively relieved oxidative stress, ameliorated inflammation by modulating microglial polarization towards anti-inflammation phenotype, and reduced hyperphosphorylated tau protein levels. Furthermore, Fe-GA CPNs treatment significantly improved cognitive function in tauopathy-induced AD rats, and achieved a neuroprotective effect against AD pathology. This study highlights the potential of coordination polymer nanoparticles as promising therapeutic candidates for AD and other tau-related neurodegenerative diseases.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biomaterials
工程技术-材料科学:生物材料
CiteScore
26.00
自引率
2.90%
发文量
565
审稿时长
46 days
期刊介绍:
Biomaterials is an international journal covering the science and clinical application of biomaterials. A biomaterial is now defined as a substance that has been engineered to take a form which, alone or as part of a complex system, is used to direct, by control of interactions with components of living systems, the course of any therapeutic or diagnostic procedure. It is the aim of the journal to provide a peer-reviewed forum for the publication of original papers and authoritative review and opinion papers dealing with the most important issues facing the use of biomaterials in clinical practice. The scope of the journal covers the wide range of physical, biological and chemical sciences that underpin the design of biomaterials and the clinical disciplines in which they are used. These sciences include polymer synthesis and characterization, drug and gene vector design, the biology of the host response, immunology and toxicology and self assembly at the nanoscale. Clinical applications include the therapies of medical technology and regenerative medicine in all clinical disciplines, and diagnostic systems that reply on innovative contrast and sensing agents. The journal is relevant to areas such as cancer diagnosis and therapy, implantable devices, drug delivery systems, gene vectors, bionanotechnology and tissue engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


