酸性气体的吸收:2-(2-二乙基氨基乙氧基)乙醇和1,3-二甲基-2-咪唑烷酮水溶液热力学和动力学方面的评价
IF 3.8
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
摘要
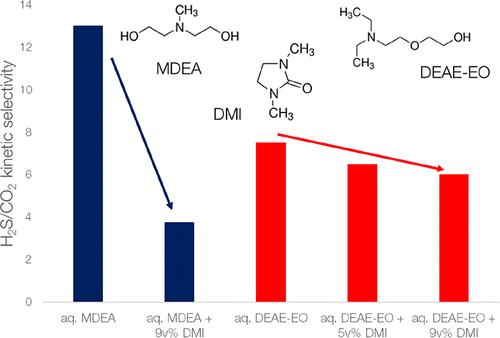
通过烷醇胺水溶液的吸收去除气体中的 CO2 和 H2S 是一个能源密集型过程。添加物理共溶剂可能会降低再生能量,但同时也会影响酸性气体吸收的热力学和动力学。在这项工作中,我们研究了在 2-(2-二乙氨基乙氧基)乙醇(DEAE-EO)水溶液中加入 1,3-二甲基-2-咪唑烷酮(DMI)对 CO2 和 H2S 吸收的热力学和动力学的影响。比较了 DMI 对水甲基二乙醇胺 (MDEA) 溶剂的影响。在所有情况下,DMI 都降低了酸性气体的溶解度和吸收率。DMI 还降低了 H2S/CO2 的初始动力学选择性,不过这种影响在水性 MDEA 中似乎比在水性 DEAE-EO 中更为明显。在有 DMI 的情况下,平衡热力学 H2S/CO2 选择性更高。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Absorption of Acid Gases: Evaluation of Thermodynamic and Kinetic Aspects of an Aqueous Solution of 2-(2-Diethylaminoethoxy) Ethanol and 1,3-Dimethyl-2-imidazolidinone
The removal of CO2 and H2S from a gas by absorption with an aqueous alkanolamine solution is an energy-intensive process. The addition of a physical cosolvent might lower the regeneration energy but also affects the thermodynamics and kinetics of acid gas absorption. In this work, we study the impact on the thermodynamics and kinetics of CO2 and H2S absorption of the addition of 1,3-dimethyl-2-imidazolidinone (DMI) to the aqueous 2-(2-diethylaminoethoxy)ethanol (DEAE-EO) solvent. A comparison is made with the impact of DMI on an aqueous methyldiethanolamine (MDEA) solvent. In all cases, DMI reduced the solubility and the absorption rate of the acid gases. DMI also reduced the initial kinetic H2S/CO2 selectivity, although this effect seems much more pronounced in aqueous MDEA than in aqueous DEAE-EO. The equilibrium thermodynamic H2S/CO2 selectivity is higher in the presence of DMI.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


