利用流感病毒诱导的免疫原性细胞死亡膜修饰聚乳酸乙醇酸纳米颗粒的肿瘤疫苗
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
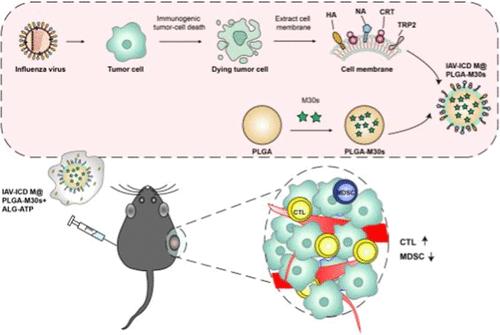
肿瘤细胞的免疫原性细胞死亡(Immunogenic cell death, ICD)以释放免疫刺激的“找我”和“吃我”信号、表达促炎细胞因子和提供个性化和广谱肿瘤抗原为特征,在肿瘤疫苗的开发中越来越受到关注。在本研究中,我们旨在研究流感病毒(IAV)是否足以在肿瘤细胞中诱导ICD,以及对IAV成分(如血凝素(HA))的额外修饰是否有助于ICD诱导的细胞产生强大的抗肿瘤作用;此外,为了评估模拟ICD免疫刺激机制的膜工程聚乳酸乙醇酸纳米颗粒(PLGA NPs)是否有潜力成为一种有希望的候选疫苗,我们用反向遗传系统拯救的IAV感染小鼠黑色素瘤细胞系(B16-F10细胞),并分别使用制备的细胞和膜修饰的PLGA NPs免疫黑色素瘤小鼠。IAV感染的肿瘤细胞呈现死亡状态,释放高迁移率组盒1 (HMGB1)和三磷酸腺苷(ATP),暴露钙网蛋白(CRT)、IAV血凝素(HA)和肿瘤抗原如酪氨酸酶相关蛋白2 (TRP2)。iav诱导的ICD细胞增强了生物源碳(bmdc)在体外的迁移、抗原摄取、交叉递呈和成熟。此外,iav诱导的ICD细胞免疫可有效抑制黑色素瘤小鼠的肿瘤生长。分离的细胞膜继承了ICD细胞的免疫特性,并通过在水凝胶系统中用肿瘤特异性辅助性t细胞肽和补充ATP修饰PLGA NPs,引发了强大的抗肿瘤免疫反应。该研究表明,通过充分利用肿瘤细胞发生ICD的免疫刺激机制、IAV修饰和纳米级递送,开发基于细胞和个性化的肿瘤疫苗是一种有希望的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Tumor Vaccine Exploiting Membranes with Influenza Virus-Induced Immunogenic Cell Death to Decorate Polylactic Coglycolic Acid Nanoparticles
Immunogenic cell death (ICD) of tumor cells, which is characterized by releasing immunostimulatory “find me” and “eat me” signals, expressing proinflammatory cytokines and providing personalized and broad-spectrum tumor antigens draws increasing attention in developing a tumor vaccine. In this study, we aimed to investigate whether the influenza virus (IAV) is efficient enough to induce ICD in tumor cells and an extra modification of IAV components such as hemeagglutinin (HA) will be helpful for the ICD-induced cells to elicit robust antitumor effects; in addition, to evaluate whether the membrane-engineering polylactic coglycolic acid nanoparticles (PLGA NPs) simulating ICD immune stimulation mechanisms hold the potential to be a promising vaccine candidate, a mouse melanoma cell line (B16–F10 cell) was infected with IAV rescued by the reverse genetic system, and the prepared cells and membrane-modified PLGA NPs were used separately to immunize the melanoma-bearing mice. IAV-infected tumor cells exhibit dying status, releasing high mobility group box-1 (HMGB1) and adenosine triphosphate (ATP), and exposing calreticulin (CRT), IAV hemeagglutinin (HA), and tumor antigens like tyrosinase-related protein 2 (TRP2). IAV-induced ICD cells enhance biomass-derived carbon (BMDCs) migration, antigen uptake, cross-presentation, and maturation in vitro. Furthermore, immunization with IAV-induced ICD cells effectively suppressed tumor growth in melanoma-bearing mice. The isolated cell membrane inherited the immunological characteristics from the ICD cells and elicited robust antitumor immune responses through decorating PLGA NPs loading with a tumor-specific helper T-cell peptide and supplemented with ATP in a hydrogel system. This study indicated a promising strategy for developing cell-based and personalized tumor vaccines through fully taking advantage of the immune stimulation mechanisms of ICD occurrence in tumor cells, IAV modification, and nanoscale delivery.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


