RuO2/CeO2催化剂对含so2烟气氧化Hg0的协同改进:理论与实验研究
IF 13.2
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
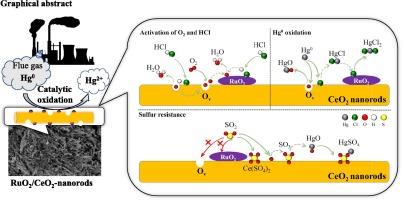
催化氧化法是一种很有前途的去除燃煤电厂单质汞(Hg0)的技术。在本研究中,制备了负载RuO2的CeO2纳米棒(CeO2- r)和CeO2纳米棒(CeO2- p),研究了它们在含二氧化硫烟气中的氧化性能。通过密度泛函理论计算,评价了不同烟气条件下Hg0氧化反应机理。结果表明,CeO2-R对Hg0的氧化效率远高于CeO2-P。负载RuO2的CeO2-R催化剂(RuO2/CeO2-R)在100-200 °C范围内表现出优异的氧化效率,氧化效率超过90 %,在200 °C时氧化效率最高,达到97.13 %。同时,RuO2/CeO2-R催化剂与CeO2-R催化剂相比,表现出更强的抗硫性能,在500 ppm SO2下保持91.10 %的氧化效率。理论计算表明,活性氧(Oa)是由CeO2(110)表面氧空位(Ov)处的分子氧(O2)生成的,HCl的存在加速了这一过程。随后,Oa促进了RuO2(110)表面HCl的活化,从而促进了Hg0的整体氧化过程。此外,与CeO2(110)-Ov和RuO2(110)表面的Ov位点相比,CeO2(110)桥位对SO2的吸附能更高,表明CeO2(110)桥位可以有效防止Hg0氧化催化剂活性位点的硫中毒。该研究揭示了RuO2/CeO2催化剂对Hg0氧化和抗硫的协同作用,为燃煤烟气中单质汞的催化氧化提供了潜在的应用前景。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Synergistic improvement of RuO2/CeO2 catalysts on Hg0 oxidation from SO2-containing flue gas: A theoretical and experimental study
Catalytic oxidation has been a promising technique for removing elemental mercury (Hg0) in coal-fired power plants. In this study, RuO2 loaded CeO2 nanorods (CeO2-R) and CeO2 nanoparticles (CeO2-P) were prepared to investigate their Hg0 oxidation performance in SO2-containing flue gas. Reaction mechanisms of Hg0 oxidation under different flue gas were evaluated through density functional theory calculations. The results show that CeO2-R had a much higher Hg0 oxidation efficiency than that of CeO2-P. The RuO2 loaded CeO2-R catalyst (RuO2/CeO2-R) exhibited superior Hg0 oxidation efficiency exceeding 90 % within the temperature range of 100–200 °C, with the maximum efficiency of 97.13 % at 200 °C. Meanwhile, the RuO2/CeO2-R catalyst displayed an enhanced sulfur resistance compared to the CeO2-R catalyst, preserving 91.10 % oxidation efficiency with 500 ppm SO2. Theoretical calculations indicate that active oxygen (Oa) was generated from molecular oxygen (O2) at oxygen vacancies (Ov) on the CeO2(110) surface, a process accelerated by the presence of HCl. Subsequently, the Oa promoted the activation of HCl on the RuO2(110) surface, thereby facilitating the overall oxidation process of Hg0. Furthermore, the higher adsorption energy of SO2 at the CeO2(110) bridge sites, compared to the Ov sites of CeO2(110)-Ov and the RuO2(110) surface, suggests that the CeO2(110) bridge sites can effectively prevent sulfur poisoning of the active sites on catalysts involved in Hg0 oxidation. This study reveals an improved synergistic effect of RuO2/CeO2 catalysts on Hg0 oxidation and sulfur resistance, providing potential application in the catalytic oxidation of elemental mercury from coal-fired flue gas.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


