铁死亡和细胞凋亡途径的机制研究:环氧康唑和黄曲霉毒素B1对斑马鱼多器官毒性的协同效应和跨代效应
IF 11.4
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
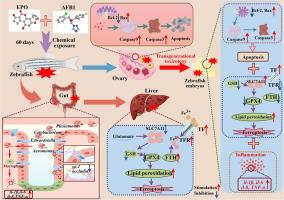
在环境中,真菌毒素和杀菌剂经常共存,可能对生物体造成协同风险。环氧康唑(Epoxiconazole, EPO)和黄曲霉毒素B1(黄曲霉毒素B1, AFB1)分别是常见的杀菌剂和真菌毒素,它们广泛存在于环境中,一旦进入机体就会对多个器官产生毒性作用,但共同暴露是否具有协同毒性作用尚不清楚。目的本研究通过多组学方法探讨EPO和AFB1共同暴露的分子机制,强调雌性斑马鱼(F0代)的多器官毒性和对后代胚胎(F1代)的潜在跨代影响。结果表明,EPO或AFB1单独或联合暴露均可加重肠道病理损伤,降低紧密连接蛋白的表达,改变肠道菌群组成,诱发肠道炎症,其中共暴露的影响更为严重。RNA-seq分析显示,F0斑马鱼肝脏和卵巢中铁下垂和凋亡通路富集。共暴露显著改变了相关分子的表达,加剧了这些器官的病理损伤。分子对接研究发现,AFB1与Caspase3、GPX4和IL-1β的结合能较EPO低,提示其可能具有更高的结合能力。此外,在未暴露的F1胚胎中,单独暴露和联合暴露都改变了与细胞凋亡、炎症反应和铁凋亡相关的分子的表达,而共同暴露显示出更显著的生物学效应,从而证实了跨代毒性。结论本研究为暴露联合诱导多器官毒性的潜在机制提供了初步证据,强调了肝脏铁下垂和卵巢凋亡是主要途径。这些发现为多种环境污染物的风险评价提供了新的视角和方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Mechanistic insights into ferroptosis and apoptosis pathways: Synergistic effects of multi-organ toxicity and transgenerational effects induced by co-exposure of epoxiconazole and aflatoxin B1 in zebrafish
Introduction
In the environment, mycotoxins and fungicides frequently coexist, potentially causing synergistic risks to organisms. Epoxiconazole (EPO) and aflatoxin B1 (AFB1) are a common fungicide and mycotoxins, respectively, which are widely present in the environment and have toxic effects on multiple organs once entering the organism, but it is still unclear whether the co-exposure has a synergistic toxic effect.Objectives
This study delves into the molecular mechanisms underlying the co-exposure to EPO and AFB1, emphasizing multi-organ toxicity in female zebrafish (F0 generation) and potential transgenerational impacts on the offspring embryos (F1 generation) through multi-omics approaches.Results
Findings indicate that exposure to either EPO or AFB1, individually or combined, intensified intestinal pathological damage, decreased the expression of tight junction proteins, altered gut microbiota composition, and induced intestinal inflammation, with co-exposure causing more severe effects. RNA-seq analysis revealed an enrichment of ferroptosis and apoptosis pathways in the liver and ovaries of F0 zebrafish. Co-exposure markedly altered the expression of associated molecules, exacerbating pathological damage in these organs. Molecular docking studies revealed that AFB1 exhibited lower binding energies to Caspase3, GPX4 and IL-1β compared to EPO, suggesting that it may have a higher binding capacity. Furthermore, both single and combined exposures modified the expression of molecules related to apoptosis, inflammatory response, and ferroptosis in unexposed F1 embryos, with co-exposure demonstrating more significant biological effects, thereby confirming transgenerational toxicity.Conclusion
The present study provides preliminary evidence on the potential mechanisms of combined exposure-induced multi-organ toxicity, highlighting ferroptosis of the liver and apoptosis of the ovary as key pathways. These findings provide new perspectives and methods for risk assessment of multiple environmental pollutants.求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


