孕期母体昼夜节律决定后代的代谢可塑性
IF 27.7
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
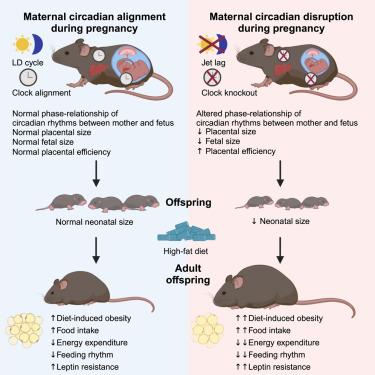
组织水平的振荡是由组织内在时钟以及源自远端器官和生物体行为的网络依赖信号实现的。然而,怀孕期间母亲的昼夜节律是否会影响胎儿节律,并影响后代对饮食挑战的长期易感性,仍未得到研究。在这里,我们证明了怀孕期间的昼夜节律中断减少了胎盘和新生儿的体重,但保留了转录和结构成熟。有趣的是,在时间紊乱母亲的后代中,饮食引起的肥胖与不规律的进食行为、下丘脑瘦素抵抗和肝脏昼夜节律重编程并行加剧。在子宫内,昼夜节律不同步改变了母亲和胎儿之间的相位关系,影响了胎盘的效率。在后代中,时间限制进食并不能完全预防肥胖,而热量限制与活动期开始的昼夜节律一致实际上改善了表型。因此,怀孕期间母亲的昼夜节律赋予了后代代谢功能的适应性特性,并为健康和疾病的发育起源提供了见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Maternal circadian rhythms during pregnancy dictate metabolic plasticity in offspring
Tissue-level oscillation is achieved by tissue-intrinsic clocks along with network-dependent signals originating from distal organs and organismal behavior. Yet, it remains unexplored whether maternal circadian rhythms during pregnancy influence fetal rhythms and impact long-term susceptibility to dietary challenges in offspring. Here, we demonstrate that circadian disruption during pregnancy decreased placental and neonatal weight yet retained transcriptional and structural maturation. Intriguingly, diet-induced obesity was exacerbated in parallel with arrhythmic feeding behavior, hypothalamic leptin resistance, and hepatic circadian reprogramming in offspring of chronodisrupted mothers. In utero circadian desynchrony altered the phase-relationship between the mother and fetus and impacted placental efficiency. Temporal feeding restriction in offspring failed to fully prevent obesity, whereas the circadian alignment of caloric restriction with the onset of the active phase virtually ameliorated the phenotype. Thus, maternal circadian rhythms during pregnancy confer adaptive properties to metabolic functions in offspring and provide insights into the developmental origins of health and disease.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell metabolism
生物-内分泌学与代谢
CiteScore
48.60
自引率
1.40%
发文量
173
审稿时长
2.5 months
期刊介绍:
Cell Metabolism is a top research journal established in 2005 that focuses on publishing original and impactful papers in the field of metabolic research.It covers a wide range of topics including diabetes, obesity, cardiovascular biology, aging and stress responses, circadian biology, and many others.
Cell Metabolism aims to contribute to the advancement of metabolic research by providing a platform for the publication and dissemination of high-quality research and thought-provoking articles.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


