SAPO-34 上高压甲醇制烯烃反应过程中水控结焦动力学
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
水作为催化剂再生的共进料和脱焦剂,越来越被认为是沸石催化甲醇制烯烃(MTO)的重要组成部分。在SAPO-34沸石催化剂上的高压MTO反应中,发现了水控焦化动力学和提高的扩散效率。通过气相色谱-质谱(GC-MS)、基质辅助激光解吸/电离傅里叶变换离子回旋共振质谱(MALDI FT-ICR MS)和紫外可见光谱(UV-vis),主要从两个方面证实了水延迟焦化的动力学行为:抑制活性烃池物质(如苯基、萘基物质)在CHA笼内老化形成多芳烃(PAHs),阻碍CHA笼间PAHs的交联。对于失活的SAPO-34催化剂,从甲醇改为水-甲醇共进料后,甲醇转化率从5%恢复到40%,高压蒸汽处理后,甲醇转化率从5%恢复到100%,进一步证实了高压水在真实MTO反应条件下的原位焦炭分解能力。此外,结构照明显微镜(SIM)提供了水影响下单个SAPO-34晶体中保留的有机物质及其时空分布的直接可视化,从而为水延迟焦化动力学和改进的扩散过程提供了视觉证据。因此,本研究揭示的水控结焦和扩散动力学机理为水相关技术在MTO行业的应用提供了重要的理论基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
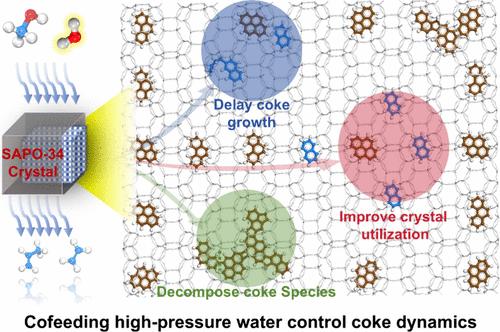
Water-Controlled Coking Dynamics during High-Pressure Methanol-to-Olefins Reaction over SAPO-34
Water, as a co-feed and decoking agent for catalyst regeneration, is increasingly recognized as a crucial component in methanol to olefins (MTO) catalysis over zeolites. In this study, water-controlled coking dynamics and improved diffusion efficiency have been revealed in a high-pressure MTO reaction over the SAPO-34 zeolite catalyst. Through gas chromatograph–mass spectrometry (GC-MS), matrix-assisted laser desorption/ionization Fourier-transform ion cyclotron resonance mass spectrometry (MALDI FT-ICR MS), and ultraviolet–visible spectroscopy (UV–vis), the kinetic behavior of water-delayed coking has been confirmed mainly in two aspects: suppressing the aging of active hydrocarbon pool species (HCPs, e.g., phenyl, naphthyl species) to form polyaromatic hydrocarbons (PAHs) within the CHA cages and hindering the cross-linking of PAHs between CHA cages. For the deactivated SAPO-34 catalyst, the restoration of methanol conversion from 5% to 40% upon switching from methanol to water–methanol co-feed and from 5% to 100% after high-pressure steam treatment further confirms the in situ coke decomposition capability of high-pressure water under the real MTO reaction conditions. Moreover, structured illumination microscopy (SIM) offers a direct visualization of the retained organic species and their spatiotemporal distribution within individual SAPO-34 crystals under the influence of water, thereby providing visual evidence for water-delayed coking dynamics and the improved diffusion process. Thus, the mechanistic insights into water-controlled coking and diffusion dynamics unveiled in this study provide a crucial theoretical foundation for the application of water-related techniques in the MTO industry.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


