用于骨质疏松症靶向治疗的含生物矿物质的仿生细胞外囊泡
IF 8.3
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
骨质疏松症(OP)是一种全身性骨骼疾病,以骨密度降低和骨折风险增高为特征。OP的治疗主要侧重于平衡骨形成和骨吸收,但增强骨质疏松骨的再矿化也是有效修复的关键策略。最近对生物矿化机制的研究强调了由成骨细胞分泌的含矿物质的细胞外囊泡(ev)在促进骨髓间充质基质/干细胞(BMSC)分化和启动基质矿化中的重要作用。根据这些原理,我们开发了一种仿生方法,通过工程设计具有骨髓归巢细胞膜(CMs)的仿生线粒体矿物质来复制成骨细胞来源的ev的结构和功能。这种以骨为目标的仿生系统具有良好的生物相容性,通过调节细胞能量代谢来促进成骨分化和刺激血管生成。此外,cm包被结构对胶原原纤维具有亲和力,有效增强了纤维内胶原矿化,从而促进骨质疏松的骨修复。总的来说,仿生系统提供了一种安全有效的治疗选择,将其定位为骨组织工程和再生医学的平台。本文章由计算机程序翻译,如有差异,请以英文原文为准。
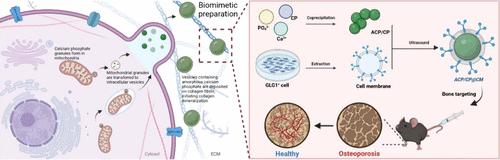
Biomimetic Extracellular Vesicles Containing Biominerals for Targeted Osteoporosis Therapy
Osteoporosis (OP) is a systemic skeletal disorder characterized by decreased bone mineral density and a heightened risk of fractures. Therapies for OP have primarily focused on balancing bone formation and bone resorption, but enhancing the remineralization of osteoporotic bone is also a key strategy for effective repair. Recent insights into biomineralization mechanisms have highlighted the essential role of mineral-containing extracellular vesicles (EVs) secreted by osteoblasts in promoting bone marrow mesenchymal stromal/stem cell (BMSC) differentiation and initiating matrix mineralization. Drawing from these principles, we developed a biomimetic approach to replicate the structure and function of the osteoblast-derived EVs by engineering biomimetic mitochondrial minerals with bone marrow homing cell membranes (CMs). This bone-targeted biomimetic system exhibits excellent biocompatibility, enhancing osteogenic differentiation and stimulating angiogenesis by regulating cellular energy metabolism. Additionally, the CM-coated structure shows affinity for collagen fibrils, effectively enhancing intrafibrillar collagen mineralization, thereby facilitating osteoporotic bone repair. Overall, the biomimetic system offers a safe and efficient therapeutic alternative, positioning it as a platform for bone tissue engineering and regenerative medicine.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


