基于气体释放纳米平台的NO/H2S气体对口腔溃疡抗菌、抗炎和镇痛特性的协同作用
IF 9.6
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
口腔黏膜伤口由于直接接触各种微生物,更容易发生炎症。这可能导致疼痛、愈合延迟和其他并发症,影响患者的日常活动,如进食和说话。因此,患者的整体生活质量显著降低。为了解决这些挑战,我们开发了一种多功能治疗纳米平台DATS@Arg-EA-SA,通过自组装胍化树突状肽(Arg-EA-SA),包裹硫化氢(H2S)供体二烯丙基三硫化物(DATS)。DATS@Arg-EA-SA富含胍的表面可以有效中和溃疡微环境中的活性氧(ROS),产生一氧化氮(NO),一氧化氮通过破坏细菌膜作为主要的抗菌药物。同时,谷胱甘肽的存在触发DATS中H2S的释放,提供补充的抗菌支持。DATS@Arg-EA-SA有效杀死所有细菌,达到与青霉素(一种经典抗生素)相当的效果。此外,它对耐甲氧西林金黄色葡萄球菌(MRSA)等耐药细菌的灭菌效果优于青霉素。在最初的抗菌阶段之后,纳米平台过渡到抗炎阶段。H2S与NO协同作用,促进M1巨噬细胞向M2巨噬细胞转化,从而降低炎症因子的表达。重要的是,H2S和NO的联合作用通过下调TRPV1和TRPV4的表达来提供有效的镇痛,从而恢复正常的饮食行为,提高整体生活质量。该系统最终促进胶原纤维沉积,加速溃疡创面的再上皮化,将DATS@Arg-EA-SA定位为临床治疗中有前景的气体输送纳米平台,用于伤口快速修复。意义声明:口腔粘膜伤口极易受到微生物感染,导致炎症、疼痛、愈合延迟和生活质量显著下降。我们开发了一种多功能治疗纳米平台(DATS@Arg-EA-SA),通过自组装的胍化树突状肽包裹H2S供体DATS,具有抗菌、抗炎和镇痛的特性。在口腔溃疡微环境中,DATS@Arg-EA-SA在ROS水平升高的情况下产生大量NO,而谷胱甘肽则触发H2S的可控释放。NO作为主要抗菌剂破坏细菌膜,H2S具有协同抗菌作用。H2S和NO协同促进巨噬细胞M1向M2转化,减轻炎症。重要的是,H2S和NO的联合作用通过下调TRPV1和TRPV4来减轻疼痛,支持正常饮食行为的恢复,提高生活质量。本文章由计算机程序翻译,如有差异,请以英文原文为准。
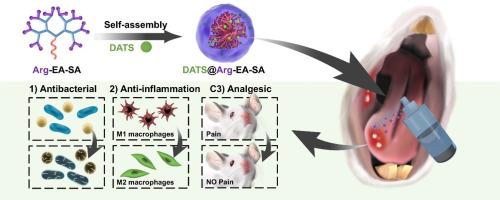
Synergistic effects of NO/H2S gases on antibacterial, anti-inflammatory, and analgesic properties in oral ulcers using a gas-releasing nanoplatform
Oral mucosal wounds are more prone to inflammation due to direct exposure to various microorganisms. This can result in pain, delayed healing, and other complications, affecting patients' daily activities such as eating and speaking. Consequently, the overall quality of life for patients is significantly reduced. To address these challenges, we developed a multifunctional therapeutic nanoplatform, DATS@Arg-EA-SA, through the self-assembly of guanidinated dendritic peptides (Arg-EA-SA) that encapsulate diallyl trisulfide (DATS), a hydrogen sulfide (H2S) donor. The guanidine-rich surface of DATS@Arg-EA-SA efficiently neutralizes reactive oxygen species (ROS) in the ulcer microenvironment, generating nitric oxide (NO), which acts as the primary antimicrobial agent by disrupting bacterial membranes. Concurrently, the presence of glutathione triggers the release of H2S from DATS, providing supplementary antibacterial support. DATS@Arg-EA-SA effectively kills all bacteria, achieving results comparable to those of penicillin, a classical antibiotic. Moreover, it demonstrates superior sterilization efficacy against drug-resistant bacteria, such as methicillin-resistant Staphylococcus aureus (MRSA), significantly outperforming penicillin. Following the initial antimicrobial phase, the nanoplatform transitions into an anti-inflammatory stage. H2S, in synergy with NO, facilitates the conversion of M1 macrophages to M2 macrophages, thereby reducing the expression of inflammatory factors. Importantly, the combination of H2S and NO provides effective analgesia by downregulating the expression of TRPV1 and TRPV4, thus restoring normal dietary behaviors and improving the overall quality of life. This system ultimately promotes collagen fiber deposition and accelerates the re-epithelialization of the ulcer wound, positioning DATS@Arg-EA-SA as a promising gas-delivery nanoplatform for rapid wound repair in the clinical treatment.
Statement of significance
Oral mucosal wounds are highly susceptible to microbial infections, leading to inflammation, pain, delayed healing, and a significant decline in quality of life. We developed a multifunctional therapeutic nanoplatform (DATS@Arg-EA-SA) via the self-assembly of guanidinated dendritic peptides encapsulating the H2S donor DATS, which exhibited antibacterial, anti-inflammatory, and analgesic properties. In the oral ulcer microenvironment, DATS@Arg-EA-SA generates substantial NO under elevated ROS levels, while glutathione triggers the controlled release of H2S. NO disrupts bacterial membranes as the primary antibacterial agent, with H2S providing synergistic antibacterial effects. Furthermore, H2S and NO synergistically promote the transformation of M1 to M2 macrophages, attenuating inflammation. Importantly, the combined action of H2S and NO alleviates pain by downregulating TRPV1 and TRPV4, supporting the restoration of normal dietary behavior and improving quality of life.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


