氨基阴离子受体的分子设计实现了长寿命氟离子电池
IF 24.4
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
阴离子受体(AAs)能够溶解金属氟盐,并在氟离子电池(FIBs)中实现可逆的氟化和除氟化。然而,大多数报道的策略只关注具有强刘易斯酸和过高氢键(HB)强度的硼基和醇基原子吸收剂,这往往导致活性物质的质量损失不可控,电解质的还原稳定性较差。虽然氨基和亚胺基团具有较好的抗还原性,但它们的HB强度显然太弱,无法解离氟化物盐。本文提出了一种在吡咯烷的五元环上引入双键和吡啶- n的分子结构设计新策略。其中,共轭效应、诱导效应和α效应协同作用,提高了亚胺基的刘易斯酸度。理论计算和实验证明1,2,4-三唑AA在提高亚胺基HB强度的同时最大程度地保持了还原稳定性。基于这一极小AA,电解质实现了前所未有的宽电化学稳定窗口(5.5 V),实现了CuF2||Pb充满电池(>;300个循环)、pb2 -Pb|| pb2 -Pb对称电池(1600 h)和pb2 ||Pb不对称电池(1600 h)的高库仑效率的氟化和除氟化的高度可逆循环。本文章由计算机程序翻译,如有差异,请以英文原文为准。
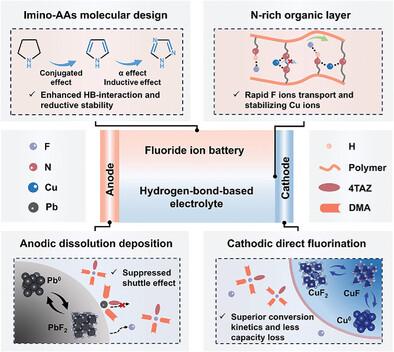
Molecular Design of Imino Anion Acceptors Enables Long-Life Fluoride Ion Batteries
Anion acceptors (AAs) enable to dissolve metal fluoride salts and achieve reversible fluorination and defluorination in fluoride ion batteries (FIBs). However, most reported strategies only focus on boron- and alcohol-based AAs with strong Lewis acidity and excessive hydrogen bond (HB) strength, which often leads to the uncontrollable mass loss of active materials and the inferior reduction stability of electrolyte. Although amino and imine groups possess preferable anti-reductive property, their HB strengths are apparently too weak to dissociate fluoride salts. Here a novel strategy is proposed for molecular structure design toward imino AAs by introducing double bonds and pyridine-N into the five-membered-ring of pyrrolidine. Therein the conjugation effect, inductive effect, and α effect are synergistically utilized to enhance the Lewis acidity of imino group. Theoretical calculations and experiments prove that 1,2,4-triazole AA retains the reduction stability in the maximum extent while increasing the HB strength of imino group. Based on this imino AA, the electrolyte achieves an unprecedented wide electrochemical stability window (5.5 V), enabling highly reversible cycling of fluorination and defluorination for CuF2||Pb full cells (>300 cycles) with a Cu+-mediated two-step redox mechanism, for PbF2-Pb||PbF2-Pb symmetric cells (1600 h) with low overpotential, and for PbF2||Pb asymmetric cells with high coulombic efficiency.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Advanced Energy Materials
CHEMISTRY, PHYSICAL-ENERGY & FUELS
CiteScore
41.90
自引率
4.00%
发文量
889
审稿时长
1.4 months
期刊介绍:
Established in 2011, Advanced Energy Materials is an international, interdisciplinary, English-language journal that focuses on materials used in energy harvesting, conversion, and storage. It is regarded as a top-quality journal alongside Advanced Materials, Advanced Functional Materials, and Small.
With a 2022 Impact Factor of 27.8, Advanced Energy Materials is considered a prime source for the best energy-related research. The journal covers a wide range of topics in energy-related research, including organic and inorganic photovoltaics, batteries and supercapacitors, fuel cells, hydrogen generation and storage, thermoelectrics, water splitting and photocatalysis, solar fuels and thermosolar power, magnetocalorics, and piezoelectronics.
The readership of Advanced Energy Materials includes materials scientists, chemists, physicists, and engineers in both academia and industry. The journal is indexed in various databases and collections, such as Advanced Technologies & Aerospace Database, FIZ Karlsruhe, INSPEC (IET), Science Citation Index Expanded, Technology Collection, and Web of Science, among others.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


