揭示氢自由基对全氟和多氟烷基物质 (PFAS) 脱氟的贡献:适用性和降解机制
IF 10.8
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
目前,全氟和多氟烷基物质(PFASs)(包括作为全氟辛酸(PFOA)和全氟辛烷磺酸替代物的全氟醚化合物)的脱氟受到氧化还原过程中产生的有效活性物质的限制。氢自由基(-H)作为一种伴生活性物质,在全氟辛烷磺酸的光氧化还原和电催化降解过程中的贡献一直被忽视。在此,我们证明了全氟羧酸和全氟醚化合物(如全氟辛酸和六氟环氧丙烷二聚酸(GenX))在紫外线照射下通过全氟烷基自由基活化水持续产生-H,从而在不使用任何试剂和催化剂的情况下实现了近乎完全的光降解和有效脱氟。重要的是,地表水中的初始溶解氧、H+和杂质对全氟辛烷磺酸的脱氟几乎没有抑制作用。通过将理论计算与靶向和非靶向分析方法相结合,阐明了 PFOA 和 GenX 在 -H 作用下的脱氟机理差异。对不同全氟辛烷磺酸光降解的研究表明,与其他化合物相比,全氟醚化合物不易通过-H的还原作用进行光降解,而部分F原子被Cl取代的多氟化合物则更容易被消除。然而,紫外线/-H 系统对全氟磺酸无效。这项研究为进一步开发全氟辛烷磺酸的去除技术和设计易于消除的全氟辛烷磺酸替代品提供了前所未有的视角。本文章由计算机程序翻译,如有差异,请以英文原文为准。
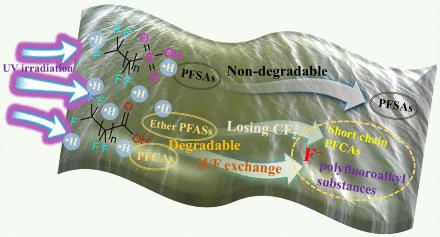
Unveiling the Contribution of Hydrogen Radicals to Per- and Polyfluoroalkyl Substances (PFASs) Defluorination: Applicability and Degradation Mechanisms
At present, the defluorination of per- and polyfluoroalkyl substances (PFASs), including perfluoroether compounds as substitutes of perfluorooctanoic acid (PFOA) and perfluorooctanesulfonate, is limited by the effective active species produced during the oxidation–reduction process. The contribution of the hydrogen radical (•H) as a companion active substance in the photoreduction and electrocatalytic degradation of PFASs has been neglected. Herein, we demonstrate that perfluorocarboxylic acids and perfluoroether compounds such as PFOA and hexafluoropropylene oxide dimer acid (GenX) underwent near-complete photodegradation and effective defluorination by continuously generating •H through perfluoroalkyl radical activation of water under UV irradiation without any reagents and catalysts. Importantly, the initial dissolved oxygen, H+, and impurities in surface water scarcely inhibited the defluorination of the PFASs. The difference in the defluorination mechanism between PFOA and GenX under the action of •H was elucidated by combining theoretical calculations with targeted and nontargeted analysis methods. The investigation of the photodegradation of different PFASs indicates that perfluoroether compounds were not easily photodegraded via reduction of •H compared with other compounds, whereas polyfluorinated compounds in which some F atoms were replaced with Cl were more prone to elimination. However, the UV/•H system was ineffective against perfluorosulfonic acids. This study provides an unprecedented perspective for further development of the removal technology of PFASs and the design of alternative PFASs that are easy to eliminate.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

环境科学与技术
环境科学-工程:环境
CiteScore
17.50
自引率
9.60%
发文量
12359
审稿时长
2.8 months
期刊介绍:
Environmental Science & Technology (ES&T) is a co-sponsored academic and technical magazine by the Hubei Provincial Environmental Protection Bureau and the Hubei Provincial Academy of Environmental Sciences.
Environmental Science & Technology (ES&T) holds the status of Chinese core journals, scientific papers source journals of China, Chinese Science Citation Database source journals, and Chinese Academic Journal Comprehensive Evaluation Database source journals. This publication focuses on the academic field of environmental protection, featuring articles related to environmental protection and technical advancements.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


